ISSN: 1659-455X • e-ISSN: 1659-407X
Vol. 16 (2), julio-diciembre 2024
Recepción 23 julio 2024 • Corregido 23 octubre 2024 • Aceptado 31 octubre 2024
DOI: https://dx.doi.org/10.15359/revmar.16-2.5
 |
Natural light vs artificial light. Effects of light pollution on the bioluminescence of dinoflagellate Pyrocystis lunula Luz natural vs. luz artificial. Efectos de la contaminación lumínica sobre la bioluminiscencia de dinoflagelado Pyrocystis lunula Davide Di Bari*1 |
ABSTRACT
Although there are thousands of marine bioluminescent species, very little is known about the effects of artificial light at night (ALAN) on these organisms, particularly those living near the sea surface, such as dinoflagellates. These organisms have a circadian clock that influences their rhythmic physiology, including processes like photosynthesis and nitrogen metabolism, which help regulate marine carbon and nitrogen cycles, respectively. The purpose of this study is to partially address this knowledge gap and research the effects of light pollution on the bioluminescent dinoflagellate Pyrocystis lunula through a series of experiments aimed at verifying the consequences due to changes in the normal day-night circadian cycle and exposure to different types of light source, colors, and light intensities. The response variable was the Corrected Total Algal Bioluminescence, which was recorded with a digital camera and then calculated with the ImageJ software. Results show that dinoflagellates do not appear to be susceptible to slight changes in the light/dark cycle. However, a total absence of light and darkness leads to a drastic inhibition of their bioluminescence, particularly under white LED or incandescent artificial light and with a light intensity of 100 lux or higher.
Keywords: bioluminescence, color, intensity, light pollution, light source
RESUMEN
Aunque hay miles de especies bioluminiscentes marinas, se sabe muy poco sobre los efectos que la luz artificial durante la noche puede tener en estos organismos, especialmente en aquellos que viven cerca de la superficie del mar, como los dinoflagelados, cuyo reloj circadiano impulsa su fisiología rítmica, como la fotosíntesis y el metabolismo del nitrógeno, que ayuda a regular el ciclo del carbono marino y el nitrógeno, respectivamente. El objetivo de esta investigación es llenar parcialmente, este vacío de conocimiento e investigar los efectos de la contaminación lumínica sobre el dinoflagelado bioluminiscente Pyrocystis lunula a través de una serie de experimentos destinados a verificar las consecuencias debidas a los cambios del ciclo día-noche (ciclo circadiano) y la exposición a diferentes tipos de fuente luminosa, colores e intensidades de luz. La variable de respuesta fue la Bioluminiscencia Total de Algas Corregida, grabada con una cámara digital y luego calculada con el software ImageJ. Los resultados muestran que los dinoflagelados no parecen ser susceptibles a ligeros cambios en el ciclo luz/oscuridad, pero una ausencia total de luz y oscuridad conduce a una inhibición drástica en su bioluminiscencia, especialmente bajo led blanco o luz artificial incandescente y con una intensidad de luz de 100 lux o mayor.
Palabras clave: bioluminiscencia, color, contaminación lumínica, fuente de luz, intensidad
Bioluminescence is the light emission by living organisms, predominantly occurring in marine creatures, from bacteria to large deep-sea vertebrates (Haddock et al. 2010). Some of the most relevant bioluminescent organisms are dinoflagellates, which have a cosmopolitan distribution (Sherr & Sherr, 2007), are vital to marine ecosystems, significantly contribute to primary production, and serve as the foundation for numerous marine food networks (Cohen et al. 2021).
In particular, Pyrocystis lunula is a photoautotrophic dinoflagellate that serves as a model organism due to its bioluminescent ability, which is related to circadian rhythms (Fajardo et al. 2020) and diel vertical migration within the water column (Hastings, 2013). P. lunula spends the day in deeper waters where nitrogen, a fundamental nutrient for its growth, is more abundant (Bhovichitra & Swift, 1977), and it is in this phase when its photosynthetic activity reaches its peak (Hastings, 2013). It spends the night in shallower waters, and during this phase, its bioluminescence is more pronounced, emitting light predominantly at the blue wavelength (Morin, 1983). Bioluminescence in dinoflagellates may have an antipredatory purpose (Esaias & Curl, 1972): to scare predatory crustaceans such as copepods, warn predators of potential toxicity, and attract the attention of higher-order visual predators to the position of copepods (Hanley & Widder, 2017).
Almost all organisms, including humans, have evolved following a natural alternation between day and night, called circadian rhythm (Hölker et al. 2010b), which is also fundamental in the regulation of vital functions of dinoflagellates (Roenneberg & Morse, 1993). An endogenous circadian system represents a complex “internal clock” in the body, which keeps synchronized with the natural day and night cycle through stimuli such as sunlight or temperature (Vitaterna et al. 2001). Organisms can better capitalize on the available environmental resources by anticipating and preparing for regular environmental changes (Corfitsen, 1996).
Other various physicochemical factors can influence the bioluminescence of marine microalgae. The availability of nutrients, such as nitrogen and phosphorus, can affect the growth and bioluminescence of microalgae. Excess nutrients can lead to algal blooms, which may alter light production (Heisler et al. 2008). Water temperature can influence the reaction rates of metabolic pathways involved in bioluminescence. Optimal temperatures promote bioluminescence, while extreme temperatures can inhibit light production (Rabha et al. 2021). Water salinity can affect microalgae physiology, impacting their ability to emit light. Changes in salinity can alter cellular functions and metabolism (Park et al. 2024). Extreme pH levels influence chemical reactions within algal cells. An optimal pH is essential for the enzymatic activities involved in bioluminescence (Craig et al. 2003). Oxygen concentration in the water can influence bioluminescence since some biochemical reactions require oxygen. Hypoxic conditions can reduce bioluminescent activity (Lambrechts et al. 2014).
Artificial light at night (ALAN) can also affect bioluminescent marine bacteria, which play a crucial ecological role in marine ecosystems. Artificial light can suppress the expression of genes responsible for bioluminescence in some bacteria. This reduction in light emission is critical for communication and defense mechanisms (Love & Prescher, 2020). Many bioluminescent bacteria form symbiotic relationships with marine organisms, such as fish. Light pollution can interfere with these connections, affecting the growth and survival of species involved (Arroyo et al. 2024). Nighttime lighting can alter the population dynamics of bioluminescent bacteria, influencing their ecological interactions and their role in the marine carbon cycle (Wang, 2018). Artificial light can also induce environmental stress in bacteria, affecting their growth and metabolism. This stress can compromise their ability to compete with other microbial species (Sathish et al. 2023).
In the last decades, artificial light at night has grown globally (Davies & Smith, 2018) with an annual increase of 6% (Hölker et al. 2010b). More than 80% of the world’s population is affected by light pollution (Falchi et al. 2016), which represents the alteration of natural lighting levels in the nocturnal environment due to human introduction of light sources (Falchi et al. 2011). Artificial light at night has been introduced to prolong daylight into the dark hours, allowing for a broader range of human activities typically performed during the day (Gaston et al. 2015). In addition, as new population centers and infrastructures are built, outdoor lighting continues to grow. Nighttime lighting is utilized to illuminate roads, bridges, airports, commercial and industrial buildings, parking areas, sports facilities, and residential neighborhoods (Falchi et al. 2011). It is important to highlight that this has dramatically influenced our well-being, boosting the likelihood of engaging in productive work, recreational activities, socializing, and fostering community ties (Gaston et al. 2015).
However, artificial nighttime lighting exhibits disadvantages of immediate interest, such as economic drawbacks. Substantial quantities of energy are necessary to maintain ALAN, which also leads to significant carbon dioxide emissions and other greenhouse gases (Gaston et al. 2015). Grid-based electric lighting is estimated to represent 195 percent of electricity production, consuming energy that releases 1 900 Mt of CO2 annually. The total cost of lighting each year is around 360 billion dollars, encompassing energy, labor, and equipment expenses (International Energy Agency, 2006).
Over time, humans have tried to find increasingly energy-efficient light sources. Specifically, after the global financial crisis and the intense pressure on public spending, local, regional, and national governments in many countries have tried to reduce the cost of public lighting (Gaston et al. 2015). Recently, there has been a considerable transition from narrow-spectrum light sources, such as High-Pressure Sodium (HPS) and Low-Pressure Sodium (LPS) lamps that mainly emit yellow or amber light, to broader-spectrum white sources like Metal Halide (MH) lamps and Light Emitting Diodes (LEDs) (Gaston et al. 2012). In particular, switching to LEDs has become increasingly appealing, as they are particularly suited to operate at variable brightness and can be turned off at times of low demand; in fact, they operate at full effectiveness without experiencing prolonged heating times (Gaston et al. 2012). Additionally, LEDs offer better control of night lighting through centralized control systems and the transition to whiter lighting with a consequent improvement in color rendering for human vision (Gaston et al. 2015).
The rise of LEDs has also resulted in many negative health consequences for humans and other organisms (Davies & Smith, 2018). In fact, in terrestrial environments, about 30% of vertebrates and 60% of invertebrates are nocturnal organisms (Hölker et al. 2010a), for which a wide variety of impacts caused by ALAN (biochemical, physiological, behavioral, intra– and interspecific interactions) have already been documented at the level of individual organisms, populations and communities belonging to the most disparate taxonomic groups (Gaston et al. 2015).
Light pollution also affects 22% of coastal marine areas (Davies et al. 2014) due to the presence of over 3 350 coastal cities globally (Depledge et al. 2010), which means that different types of light sources such as housing, walks, piers, ports, and lighthouses (Garratt et al. 2019) brightly illuminate long stretches of coastline (Luijendijk et al. 2018). Several adverse effects of ALAN pollution on coastal marine organisms are known: temporal desynchronization of gamete release in corals (Kaniewska et al. 2015), alteration of predation in gastropods (Manríquez et al. 2021) and mobility in sea urchins (Di Bari, 2023), reduction of growth rate in amphipods (Luarte et al. 2016) and reproductive success in fish (Fobert et al. 2019), fatal collisions in seabirds (Rodríguez et al. 2017) and disorientation of hatchlings of sea turtles (Price et al. 2018). However, while in the terrestrial environment, the effects of light pollution on bioluminescent organisms, such as fireflies, are known (Owens et al. 2022), influencing their reproduction (Costin & Boulton, 2016; Kivelä et al. 2023), development (Owens & Lewis, 2021), and predator-prey interactions (Firebaugh & Haynes, 2019), very little is known about the effects of ALAN on the coastal marine bioluminescent organisms.
This study aims to fill this knowledge gap and explore the effects of ALAN on the bioluminescent dinoflagellate Pyrocystis lunula through a series of experiments intended to verify the consequences of changes in the normal day-night circadian cycle and exposure to different types of light sources, colors, and light intensities.
Cultures of the marine bioluminescent dinoflagellate Pyrocystis lunula were grown at a temperature of 21°C, a salinity of 31 PSU, a pH of 8.2, and f/2 Medium as the nutrient supplement (Guillard, 1975) under a natural 12h/12h light/dark cycle for a week (exponential phase) (Fajardo et al. 2020). Dinoflagellate P. lunula emitted short flashes of blue light of approximately 100 ms in duration (Latz & Rohr, 2005), with a wavelength of 474 nm (Watanabe & Tanaka, 2011), which were stimulated mechanically by fluid shear stress (Latz et al. 2004) using a vortex mixer (TX4 Digital Vortex Mixer) at 2 000 rpm on 40 ml vials. Each experiment lasted ten nights (for a total period from January 10 to February 18, 2024) with a measurement per experimental condition each time, after 3 h into the dark phase when cells were fully dark-adapted and at the maximum bioluminescence capacity (Lindström et al. 2017).
A Canon PowerShot G7 X Mark II digital camera was used to take photos of algae bioluminescence, setting the ISO speed to 10 000 and having a wide aperture (f/2.8) to allow maximum light to hit the sensor (Fig. 1). Algal bioluminescence was quantified using ImageJ, a free and open-source software for processing and analyzing pixel intensity of scientific images. The response variable was the Corrected Total Algal Bioluminescence (CTAB), calculated with the following formula: CTAB = Integrated Algal Density - (Algal bioluminescence area · Background pixel intensity).
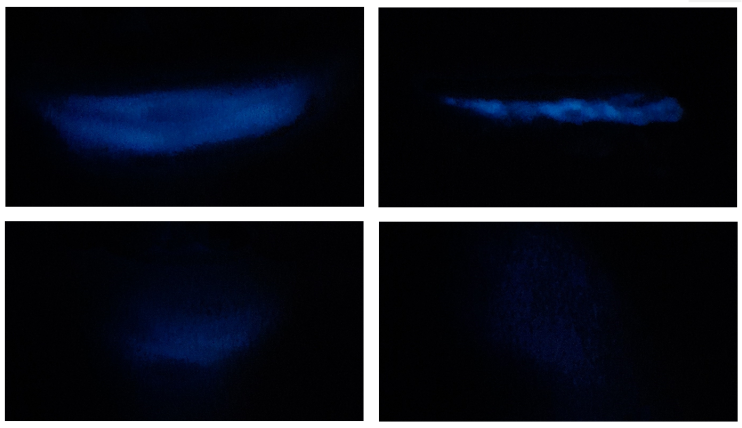
Fig. 1. Examples of dinoflagellate bioluminescence after mechanical stimulation
Fig. 1. Ejemplos de bioluminiscencia dinoflagelada tras estimulación mecánica
The first experiment aims to evaluate the possible effects of changes in the typical day-night circadian cycle on the bioluminescent dinoflagellate Pyrocystis lunula with different experimental conditions (12:12, 18:6, 6:18, 24:0, and 0:24 h of light/dark cycle), achieved either by extending the period of natural light with artificial light or by moving dinoflagellates into a dark room, to verify how high the stress level is induced and measured as inhibition of bioluminescent function.
The second experiment aims to evaluate differences in the possible effects of various types of light sources (light-emitting diode, incandescent, gas-discharge, and organic light-emitting diode) at the same light intensity (100 lux) to test for differences in bioluminescence inhibition during a 12 h night period.
The third experiment aims to evaluate differences in the possible effects of various light intensity values (0, 0.5, 5, 25, 100, and 500 lux), similar to those found in actual conditions under the same light source, an ordinary steady white LED lamp, to test for differences in bioluminescence inhibition during a 12 h night period.
The fourth experiment aims to evaluate differences in the possible effects of different colors (red, orange, yellow, green, blue, and white) with the same type of light source (steady LED lamp) and light intensity (100 lux) during a 12-hour night period. In addition, changes between a steady light source and a blinking light have also been tested using a white LED lamp.
The experimental design included a comparison between the experimental conditions using the Analysis of Variance (ANOVA) after verifying the observance of the assumptions (normality through the Shapiro–Wilk test and equality of variances through Levene’s test). Data analysis and graph plotting were conducted using the statistical software R 4.2.3, considering P < 0.05.
The bioluminescence rhythm of many light-emitting dinoflagellate species is prominent (Valiadi & Iglesias-Rodríguez, 2013), is regulated at the translational level (Hastings, 2007), and shows no resemblance to any known model eukaryotic or prokaryotic clock architecture (Jadhav et al. 2022). For example, in Lingulodinium polyedrum, the regulation of bioluminescent activity is allowed by a daily synthesis and destruction of proteins (Dunlap & Hastings, 1981), while the amino acids from the degradation are conserved and used in the subsequent cycle (Johnson et al. 1984). Contrarily, in Pyrocystis lunula, there is no degradation and resynthesis of luciferase (Knaust et al. 1998); instead, the position of the luminous organelles emitting light varies from day to night (Colepicolo et al. 1993).
The results of the statistical analysis of the four experimental conditions of different light/dark cycles (Fig. 2), through the unidirectional ANOVA, show significant differences between at least two averages of the analyzed groups (F = 594). Subsequently, to know how many and which groups had statically different averages, unplanned multiple comparisons were conducted using a post–hoc test (Tukey–Kramer), which showed significant differences between the following groups: q 0:24/6:18 = 39.71, q 0:24/12:12 = 48.95, q 0:24/18:6 = 35.73, q 0:24/24:0 = 4.13, q 6:18/12:12 = 9.23, q 6:18/24:0 = 43.85, q 12:12/18:6 = 13.22, q 12:12/24:0 = 53.08, q 18:6/24:0 = 39.86.
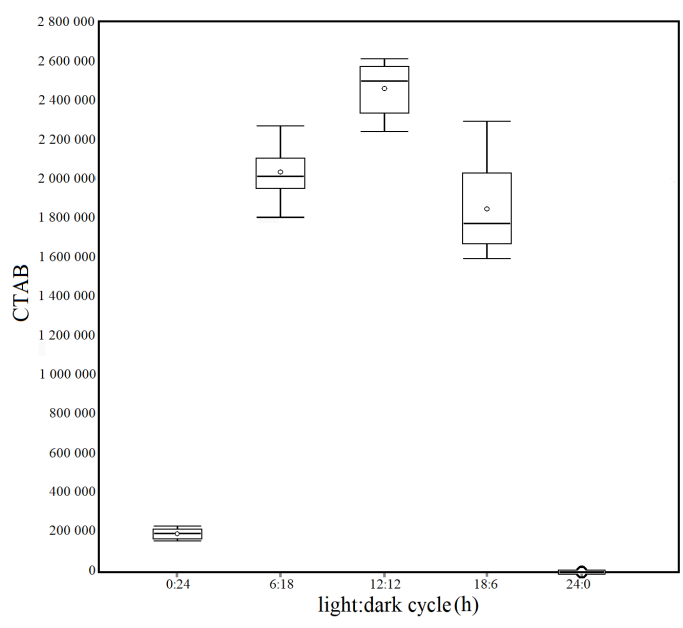
Fig. 2. Box plots of the Corrected Total Algal Bioluminescence (CTAB) in each light/dark cycle condition. Mean (± standard error): 0:24 = 191 600 ± 8 261; 6:18 = 2 032 889 ± 46 154; 12 :12 = 2 460 961 ± 43 597; 18 :6 = 1 847 988 ± 81 538; 24:0 = 0 ± 0
Fig. 2. Diagramas de caja de la bioluminiscencia total de algas corregida (CTAB, por sus siglas en inglés) en cada condición de ciclo de luz/oscuridad. Media (± error estándar): 0:24 = 191 600 ± 8 261; 6:18 = 2 032 889 ± 46 154; 12 :12 = 2 460 961 ± 43 597; 18 :6 = 1 847 988 ± 81 538; 24:0 = 0 ± 0
In the extreme cases of the light/dark cycle (0:24 and 24:0) in this first experiment, a drastic reduction in bioluminescence production is observed. In contrast, differences in intermediate conditions (6:18, 12:12, and 18:6) decrease, potentially simulating natural seasonal variations in day and night cycles. These results confirm that the bioluminescent capacity of dinoflagellate P. lunula is significantly influenced by an alternation of light and dark according to a variable, but necessarily the circadian rhythm.
Chemical reactions, heat, mass conversion, or a different frequency of electromagnetic energy are some energy sources that light sources use to produce photons (Nemade, 2023; Mandal et al. 2024). While some studies indicate that various light sources do not affect algal growth or the production of photosynthetic pigments (Bialevich et al. 2022), others claim that growth under fluorescent light outperformed growth under LED sources (Satthong et al. 2019). Even though conventional fluorescent lights based on older technology have a very irregular quantum spectrum, they proved to be better culture light sources for some algae than lighting based on LED technology (Ritchie & Sma-Air, 2023).
The results of the statistical analysis of the four experimental conditions of different light sources (Fig. 3), through the unidirectional ANOVA, show significant differences between at least two averages of the analyzed groups (F = 237). Subsequently, to know how many and which groups had statically different averages, unplanned multiple comparisons were conducted using a post–hoc test (Tukey–Kramer), which showed significant differences between the following groups: q OLED/Gas-discharge = 24.56, q OLED/LED = 32.51, q OLED/Incandescent = 32.51, q Gas-discharge/LED = 7.95, q Gas-discharge/Incandescent = 7.95).
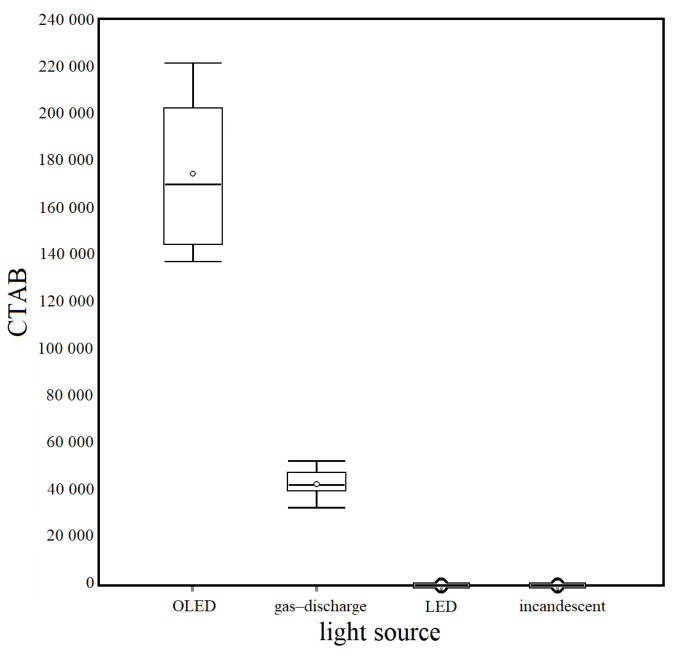
Fig. 3. Box plots of the Corrected Total Algal Bioluminescence (CTAB) in each light source condition. Mean (± standard error): Organic light-emitting diode (OLED) = 173 901 ± 10 496; Gas–discharge = 42 518 ± 2 068; Light-emitting diode (LED) = 0 ± 0; Incandescent = 0 ± 0
Fig. 3. Diagramas de la bioluminiscencia total de algas corregida (CTAB) en cada condición de fuente de luz. Media (± error estándar): diodo emisor de luz orgánico (OLED) = 173 901 ± 10 496; descarga de gas = 42 518 ± 2 068; diodo emisor de luz (LED) = 0 ± 0; incandescente = 0 ± 0
The differences in this study also show a higher CTAB under gas-discharge light sources than LEDs and incandescent lamps, probably due to the latter’s lower luminosity, which has a reduced impact on these organisms. Finally, CTAB values were much higher in the organic light-emitting diode (OLED), mainly used to create digital displays in devices such as computers, phones, and television screens (Zou et al. 2020) than in any other experimental condition. OLEDs generate diffused light in various shades that are not glaring and lack harsh shadows. However, they are still underused because of the current high cost of their technology compared to other lighting systems.
Light intensity is one of the determinants of the depth within the water column at which phytoplankton are found (Enright, 1977) with modifications of diel vertical migration (Ludvigsen et al. 2018). In particular, artificial light inhibits division rate and cell size in phototrophic dinoflagellates at high levels (Swift & Meunier, 1976), while it stimulates photosynthetic activity, growth, and ingestion rates at low levels (Jeong et al. 2018). Light intensity can also alter the consequences of fish predation on zooplankton and potentially, in turn, affect their population and community structure (Talanda et al. 2022).
The results of the statistical analysis of the control condition in the dark and the five experimental conditions of different light intensities (Fig. 4) through the unidirectional ANOVA show significant differences between at least two averages of the analyzed groups (F = 1 928). Subsequently, to know how many and which groups had statically different averages, unplanned multiple comparisons were conducted using a post–hoc test (Tukey-Kramer), which showed significant differences between the following groups: q 0/0.5 lux = 41.34, q 0/5 lux = 44.63, q 0/25 lux = 80.39, q 0/100 lux = 110.85, q 0/500 lux = 110.85, q 0.5/25 lux = 39.05, q 0.5/100 lux = 69.51, q 0.5/500 lux = 69.51, q 5/25 lux = 35.76, q 5/100 lux = 66.22, q 5/500 lux = 66.22, q 25/100 lux = 30.46, q 25/500 lux = 30.46.
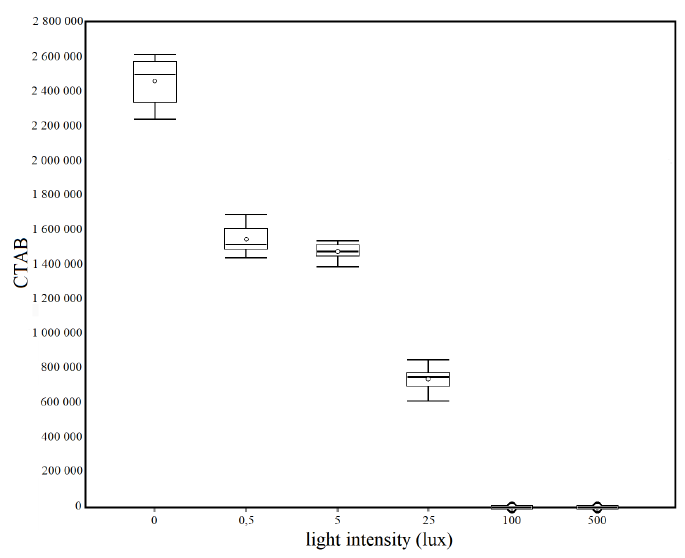
Fig. 4. Box plots of the Corrected Total Algal Bioluminescence (CTAB) in each light intensity condition. Mean (± standard error): 0 lux = 2 460 961 ± 43 597; 0.5 lux = 1 543 166 ± 25 181; 5 lux = 1 470 112 ± 16 406; 25 lux = 676 260 ± 12 385; 100 lux = 0 ± 0; 500 lux = 0 ± 0
Fig. 4. Diagramas de la bioluminiscencia total de algas corregida (CTAB) en cada condición de intensidad de luz. Media (± error estándar): 0 lux = 2 460 961 ± 43 597; 0.5 lux = 1 543 166 ± 25 181; 5 lux = 1 470 112 ± 16 406; 25 lux = 676 260 ± 12 385; 100 lux = 0 ± 0; 500 lux = 0 ± 0
Artificial light emission interferes with the dinoflagellate’s natural bioluminescence, completely inhibiting it at high intensities of 100 and 500 lux. However, low levels of artificial light intensity (0.5 and 5 lux) can also affect this function, decreasing it by more than 40%, which shows their high sensitivity to this form of pollution. The threshold value of total inhibition of bioluminescent is between 25 and 100 lux.
The efficiency of the phytoplankton’s photosynthetic activity is affected not only by light quantity, i.e., intensity but also by its quality, i.e., the availability of different wavelengths (Neun et al. 2022). Moreover, the available light quality is linked to the vertical distribution and coexistence of phytoplankton species within the water column (Stomp et al. 2008; Hickman et al. 2009), also affecting their biomass and diversity (Diamantopoulou et al. 2021).
The results of the statistical analysis of the control condition in the dark and the seven experimental conditions of different light colors (Fig. 5) through the unidirectional ANOVA show significant differences between at least two averages of the analyzed groups (F = 2 532). Subsequently, to know how many and which groups had statically different averages, unplanned multiple comparisons were conducted using a post–hoc test (Tukey-Kramer), which showed significant differences between the following groups: q blinking white/green = 5.28, q blinking white/yellow = 12.65, q blinking white/orange = 35.04, q blinking white/red = 95.81, q blinking white/dark = 127.69, q white/green = 7.91, q white/yellow = 15.28, q white/orange = 37.67, q white/red = 98.44, q white/dark = 130.32, q blue/green = 7.91, q blue/yellow = 15.28, q blue/orange = 37.67, q blue/red = 98.44, q blue/dark = 130.32, q green/yellow = 7.36, q green/orange = 29.76, q green/red = 90.53, q green/dark = 122.41, q yellow/orange = 22.39, q yellow/red = 83.17, q yellow/dark = 115.05, q orange/red = 60.78, q orange/dark = 92.66, q red/dark = 31.88.
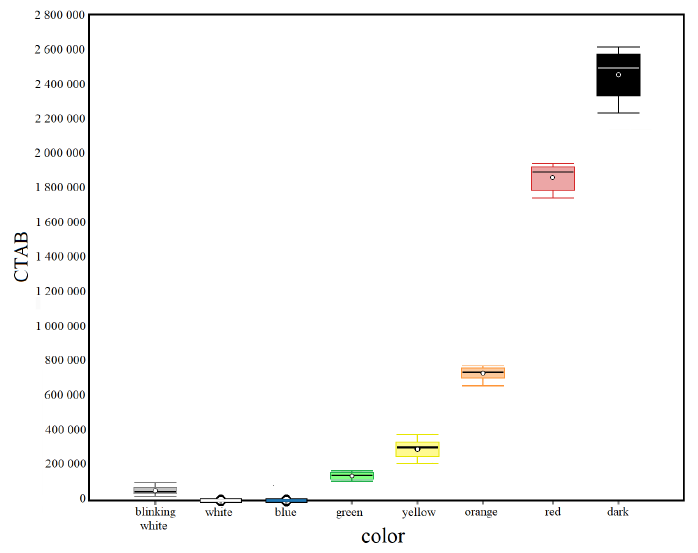
Fig. 5. Box plots of the Corrected Total Algal Bioluminescence (CTAB) in each light color condition. Mean (± standard error): Blinking White = 49 698 ± 6 307; White = 0 ± 0; Blue = 0 ± 0; Green = 149 415 ± 1 779; Yellow = 288 479 ± 17 789; Orange = 711 303 ± 9 373; Red = 1 858 968 ± 22 437; Dark = 2 460 961 ± 43 597
Fig. 5. Diagramas de la bioluminiscencia total de algas corregida (CTAB) en cada condición de color claro. Media (± error estándar): blanco intermitente = 49 698 ± 6 307; blanco = 0 ± 0; azul = 0 ± 0; verde = 149 415 ± 1 779; amarillo = 288 479 ± 17 789; naranja = 711 303 ± 9 373; rojo = 1 858 968 ± 22 437; oscuro = 2 460 961 ± 43 597
Various colors of artificial light cause a different alteration of bioluminescence with a decrease in light emitted by dinoflagellates in all experimental conditions compared to the natural condition of darkness. Results show a bioluminescence inhibition scale concerning the wave frequency of colors of the visible spectrum. In particular, a decrease in bioluminescence is observed as the wave frequency increases. Long-wavelength red light is quickly absorbed and extinguished by the upper layers of water, and most marine creatures cannot perceive red light. This is likely why red also has a limited impact on the bioluminescence of dinoflagellates.
To date, known marine bioluminescent organisms include 9 405 species, of which 2 781 are luminescent (Claes et al. 2024). The ability to emit light at night is essential for these organisms. It is equally crucial to know the effects that artificial light at night has on them, especially those living near the sea surface, such as the dinoflagellates whose circadian clock drives their rhythmic physiology, including their nitrogen metabolism and photosynthesis, which help them regulate the nitrogen cycle and marine carbon, respectively (Jadhav et al. 2022).
This paper is one of the first studies to analyze light pollution’s effects on marine bioluminescent organisms by applying a fast, innovative, and inexpensive methodological photography-based approach in this research field. Using a digital camera to measure the bioluminescence of microalgae offers several advantages compared to other techniques. First, digital cameras allow for the capture of visual images and the documenting of bioluminescent events in real time, providing both qualitative and quantitative data useful for analysis. The versatility of this type of camera enables the adjustment of settings such as ISO, aperture, and exposure time, making it easier to adapt to varying light conditions and specific experimental needs. Additionally, images can be easily analyzed and stored, simplifying the comparison of different experiments or samples. Finally, digital cameras are generally more accessible and cost-effective than specialized instruments like luminometers or spectrophotometers, making them an ideal choice for laboratories with budget constraints or for preliminary studies. This combination of practicality and functionality makes digital cameras a valuable tool in bioluminescence research.
The results of the four experiments show that dinoflagellates do not appear susceptible to slight changes in the light/dark cycle. However, a total absence of light and darkness leads to a drastic (if not total) decrease in bioluminescence, especially under white LED or incandescent artificial light and with a light intensity of 100 lux or higher. OLEDs represent a rapidly growing eco-friendly lighting source due to their extreme flexibility, mercury-free manufacture, excellent color quality, and wide viewing angle (Zou et al. 2020) and also appear to be the source with the least impact on bioluminescent organisms. However, they are still highly underused for external lighting systems in coastal areas.
This study could provide valuable insights to minimize the effects of artificial light on bioluminescent organisms and inspire further studies to enhance our understanding of this issue, particularly regarding marine species that remain partially unexplored. With the rise of urbanization and light pollution, it is essential to consider the impact of artificial light in coastal planning and conservation strategies, as these factors threaten marine biodiversity and ecosystem health. Despite progress in understanding these effects, further research is needed to quantify the impact of artificial light on various species and environmental conditions and develop effective mitigation strategies.
The author wishes to thank the anonymous reviewers for their insightful comments that enhanced this document.
Arroyo, H. L., Abascal, A., Degen, T., Aubé, M., Espey, B. R., Gyuk, G., ... & Kyba, C. C. M. (2024). Monitoring, trends and impacts of light pollution. Nat. Rev. Earth Environ., 5, 417-430. https://doi.org/10.1038/s43017-024-00555-9
Bhovichitra, M. & Swift, E. (1977). Light and dark uptake of nitrate and ammonium by large oceanic dinoflagellates: Pyrocystis lunula, Pyrocystis fusiformis, and Dissodinium lunula. Limnol. Oceanogr., 22(1), 73-83. https://doi.org/10.4319/lo.1977.22.1.0073
Bialevich, V., Zachleder, V. & Bišova, K. (2022). The Effect of Variable Light Source and Light Intensity on the Growth of Three Algal Species. Cells, 11(8), 1293. https://doi.org/10.3390/cells11081293
Claes, J. M., Haddock, S. H. D., Coubris, C. & Mallefet, J. (2024). Systematic Distribution of Bioluminescence in Marine Animals: A Species-Level Inventory. Life, 14(4), 432. https://doi.org/10.3390/life14040432
Cohen, N. R., McIlvin, M. R., Moran, D. M., Held, N. A., Saunders, J. K., Hawco, N. J., ... & Saito, M. A. (2021). Dinoflagellates alter their carbon and nutrient metabolic strategies across environmental gradients in the central Pacific Ocean. Nat. Microbiol., 6(2), 173-186. https://doi.org/10.1038/s41564-020-00814-7
Colepicolo, P., Roenneberg, T., Morse, D., Taylor, W. & Hastings, J. W. (1993). Circadian regulation of bioluminescence in the dinoflagellate Pyrocystis lunula. J. Phycol., 29(2), 173-179. https://doi.org/10.1111/j.0022-3646.1993.00173.x
Corfitsen, M. T. (1996). Enhanced tiredness among young impaired male nighttime drivers. Accid. Anal. Prev., 28(2), 155-162. https://doi.org/10.1016/0001-4575(95)00042-9
Costin, K. J. & Boulton, A. M. (2016). A field experiment on the effect of introduced light pollution on fireflies (Coleoptera: Lampyridae) in the Piedmont Region of Maryland. The Coleopt. Bull., 70(1), 84-86. https://doi.org/10.1649/072.070.0110
Craig, J. M., Klerks, P. L., Heimann, K. & Waits, J. L. (2003). Effects of salinity, pH and temperature on the re-establishment of bioluminescence and copper or SDS toxicity in the marine dinoflagellate Pyrocystis lunula using bioluminescence as an endpoint. Environ. Pollut., 125(2), 267-275. https://doi.org/10.1016/S0269-7491(03)00059-9
Davies, T. W., Duffy, J. P., Bennie, J. & Gaston, K. J. (2014). The nature, extent, and ecological implications of marine light pollution. Front. Ecol. Environ., 12(6), 347-355. https://doi.org/10.1890/130281
Davies, T. W. & Smith, T. (2018). Why artificial light at night should be a focus for global change research in the 21st century. Glob. Chang. Biol., 24(3), 872-882. https://doi.org/10.1111/gcb.13927
Depledge, M., Gordard-Codding, C. A. J. & Bowen, R. E. (2010). Light pollution in the sea. Mar. Pollut., 60(9), 1383-1385. https://doi.org/10.1016/j.marpolbul.2010.08.002
Di Bari, D. (2023). Effects of artificial light at night on the mobility of the sea urchin Paracentrotus lividus. Mar. Fish. Sci., 37(1), 41-52. https://doi.org/10.47193/mafis.3712024010106
Diamantopoulou, C., Christoforou, E., Dominoni, D. M., Kaiserli, E., Czyzewski, J., Mirzai, N. & Spatharis, S. (2021). Wavelength-dependent effects of artificial light at night on phytoplankton growth and community structure. Proc. R. Soc. B., 288, 20210525. https://doi.org/10.1098/rspb.2021.0525
Dunlap, J. C. & Hastings, J. W. (1981). The biological clock in Gonyaulax control luciferase activity by regulating turnover. J. Biol. Chem., 256(20), 10509-10518. https://doi.org/10.1016/S0021-9258(19)68651-5
Enright, J. T. (1977). Copepods in a hurry sustained high speed upward migration, Limnol. Oceanogr., 22(1), 118-125. https://doi.org/10.4319/LO.1977.22.1.0118
Esaias, W. E. & Curl, H. C. (1972). Effect of dinoflagellate bioluminescence on copepod ingestion rates. Limn. Oceanogr., 17(6), 901–906. https://doi.org/10.4319/lo.1972.17.6.0901
Fajardo, C., De Donato, C., Rodulfo, H., Martínez-Rodríguez, G., Costas, B., Mancera, J. M. & Fernández-Acero, F. J. (2020). New Perspectives Related to the Bioluminescent System in Dinoflagellates: Pyrocystis lunula, a Case Study. Int. J. Mol. Sci., 21(5), 1784. https://doi.org/10.3390/ijms21051784
Falchi, F., Cinzano, P., Elvidge, C. D., Keith, D. M. & Haim, A. (2011). Limiting the impact of light pollution on human health, environment and stellar visibility. J. Environ. Manage., 92(10), 2714-2722. https://doi.org/10.1016/j.jenvman.2011.06.029
Falchi, F., Cinzano, P., Duriscoe, D., Kyba, C. C., Elvidge, C. D., Baugh, K., ... & Furgoni, R. (2016). The new world atlas of artificial night sky brightness. Sci. Adv., 2(6), e1600377. https://doi.org/10.1126/sciadv.1600377
Firebaugh, A. & Haynes, K. J. (2019). Light pollution may create demographic traps for nocturnal insects. Basic Appl. Ecol., 34, 118-125. https://doi.org/10.1016/j.baae.2018.07.005
Fobert, E. K., Burke da Silva, K. & Swearer, S. E. (2019). Artificial light at night causes reproductive failure in clownfish. Biol. Lett., 15(7), 20190272. https://doi.org/10.1098/rsbl.2019.0272
Garratt, M. J., Jenkins, S. R. & Davies, T. W. (2019). Mapping the consequences of artificial light at night for intertidal ecosystems. Sci. Total Environ., 691, 760-768. https://doi.org/10.1016/j.scitotenv.2019.07.156
Gaston, K. J., Davies, T. W., Bennie J. & Hopkins J. (2012). Reducing the ecological consequences of nighttime light pollution: Options and developments. J. Appl. Ecol. 49(6), 1256-1266. https://doi.org/10.1111/j.1365-2664.2012.02212.x
Gaston, K. J., Gaston, S., Bennie, J. & Hopkins, J. (2015). Benefits and costs of artificial nighttime lighting of the environment. Environ. Rev., 23(1), 14-23. https://doi.org/10.1139/er-2014-0041
Guillard, R. L. L. (1975). Culture of phytoplankton for feeding marine invertebrates. In W. L. Smith & M. H. Chanley (Eds.), Culture of Marine Invertebrate Animals (pp. 29-60). USA: Springer.
Haddock, S. H. D., Moline, M. A. & Case, J. F. (2010). Bioluminescence in the Sea. Annu. Rev. Mar. Sci., 2(1), 443-493. https://doi.org/10.1146/annurev-marine-120308-081028
Hanley, K. A. & Widder, E. A. (2017). Bioluminescence in Dinoflagellates: Evidence that the Adaptive Value of Bioluminescence in Dinoflagellates is Concentration Dependent. Photochem. Photobiol., 93(2), 519-530. https://doi.org/10.1111/php.12713
Hastings, J. W. (2007). The Gonyaulax Clock at 50: Translational Control of Circadian Expression. Cold Spring Harb. Symp. Quant. Biol., 72(1), 141-144. https://doi.org/10.1101/sqb.2007.72.026
Hastings, J. W. (2013). Circadian rhythms in dinoflagellates: What is the purpose of synthesis and destruction of proteins? Microorganisms, 1(1), 26-32. https://doi.org/10.3390/microorganisms1010026
Heisler, J., Glibert, P. M., Burkholder, J. M., Anderson, D. M., Cochlan, W., Dennison, W. C., ... & Suddleson, M. (2008). Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae, 8(1), 3-13. https://doi.org/10.1016/j.hal.2008.08.006
Hickman, A. E., Holligan, P. M., Moore, C. M., Sharples, J., Krivtsov, V. & Palmer, M. R. (2009). Distribution and chromatic adaptation of phytoplankton within a shelf sea thermocline. Limn. Oceanogr., 54(2), 525-536. https://doi.org/10.4319/lo.2009.54.2.0525
Hölker, F., Wolter, C., Perkin, E. K. & Tockner, K. (2010a). Light pollution as a biodiversity threat. Trends Ecol. Evol., 25(12), 681-682. https://doi.org/10.1016/j.tree.2010.09.007
Hölker F., Moss R., Griefahn B., Kloas W., Voigt C. C., Henckel D., ... & Tockner K. (2010b). The dark side of light: a transdisciplinary research agenda for light pollution policy. Ecol. Soc., 15(4), 13. http://doi.org/10.5751/ES-03685-150413
International Energy Agency. (2006). Light’s labour’s lost: policies for energy efficient lighting. France. OECD/IEA. https://doi.org/10.1787/19900694
Jadhav, D. B., Sriramkumar, Y. & Roy, S. (2022). The enigmatic clock of dinoflagellates, is it unique? Front. Microbiol., 13, 1004074. https://doi.org/10.3389%2Ffmicb.2022.1004074
Jeong, H. J., Lee, K. H., Yoo, Y. D., Kang, N. S., Song, J. Y., Kim, T. H., ... & Potvin, E. (2018). Effects of light intensity, temperature, and salinity on the growth and ingestion rates of the red-tide mixotrophic dinoflagellate Paragymnodinium shiwhaense. Harmful Algae, 80, 46-54. https://doi.org/10.1016/j.hal.2018.09.005
Johnson, C. H., Roeber, J. & Hastings, J. W. (1984). Circadian changes in enzyme concentration account from rhythm of enzyme activity in Gonyaulax. Science, 223(4643), 1428-1430. https://doi.org/10.1126/science.223.4643.1428
Kaniewska, P., Alon, S., Kariko-Lampert, S., Hoegh-Guldberg, O. & Levy, O. (2015). Signaling cascades and the importance of moonlight in coral broadcast mass spawning. eLife, 4, e09991. https://doi.org/10.7554/elife.09991
Kivelä, L., Elgert, C., Lehtonen, T. K. & Candolin, U. (2023). The color of artificial light affects mate attraction in the common glow-worm. Sci. Total Environ., 857(3), 159451. https://doi.org/10.1016/j.scitotenv.2022.159451
Knaust, R., Urbig, T., Li, L., Taylor, W. & Hastings, J. W. (1998). The circadian rhythm of bioluminescence in Pyrocystis is not due to differences in the amount of luciferase: A comparative study of three bioluminescent marine dinoflagellates. J. Phycol., 34(1), 167-172. https://doi.org/10.1046/j.1529-8817.1998.340167.x
Lambrechts, D., Roeffaers, M., Goossens, K., Hofkens, J., Vande Velte, G., Van de Putte, T., ... & Van Oosterwyck, H. (2014). A causal relation between bioluminescence and oxygen to quantify the cell niche. PLoS One, 19, 9(5), e97572. https://doi.org/10.1371/journal.pone.0097572
Latz, M. I., Juhl, A. R., Ahmed, A. M., Elghobashi, S. E. & Rohr, J. (2004). Hydrodynamic stimulation of dinoflagellate bioluminescence: A computational and experimental study. J. Exp. Biol., 207(11), 1941-1951. https://doi.org/10.1242/jeb.00973
Latz, M. I. & Rohr, J. (2005). Glowing with the flow. Opt. Photonics News, 16(10), 40-45. http://doi.org/10.1364/OPN.16.10.000040
Lindström, J., Grebner, W., Rigby, K. & Selander, E. (2017). Effects of predator lipids on dinoflagellate defense mechanisms – increased bioluminescence capacity. Sci. Rep., 7(1), 13104. https://doi.org/10.1038/s41598-017-13293-4
Love, A. C. & Prescher, J. A. (2020). Seeing (and Using) the Light: Recent Developments in Bioluminescence Technology. Cell Chem. Biol., 27(8), 904-920. https://doi.org/10.1016/j.chembiol.2020.07.022
Luarte, T., Bonta, C. C., Silva-Rodríguez, E. A., Quijón, P. A., Miranda, C., Farias, A. A. & Duarte C. (2016). Light pollution reduces activity, food consumption and growth rates in a sandy beach invertebrate. Environ. Pollut., 218, 1147-1153. https://doi.org/10.1016/j.envpol.2016.08.068
Ludvigsen, M., Berge, J., Geoffroy, M., Cohen, J. H., De La Torre, P. R., Nornes, S. M., ... & Johnsen, G. (2018). Use of an Autonomous Surface Vehicle reveals small-scale diel vertical migrations of zooplankton and susceptibility to light pollution under low solar irradiance. Sci. Adv., 4(1), eaap9887. https://doi.org/10.1126/sciadv.aap9887
Luijendijk, A., Hagenaars, G., Raasinghe, R., Baart, F., Donchyts, G. & Aarninkhof, S. (2018). The State of the World’s Beaches. Sci. Rep., 8(1), 6641. https://doi.org/10.1038/s41598-018-24630-6
Mandal, G., Bauri, J. & Choudhary, R. B. (2024). Conjugated polymeric nanocomposite-based light-generating active materials for OLED applications: A review. Mater. Sci. Eng. B, 303, 117271. https://doi.org/10.1016/j.mseb.2024.117271
Manríquez, P. H., Jara, M. E., González, C. P., Seguel, M., Quijón, P. A., Widdicombe, S., ... & Duarte, C. (2021). Effects of artificial light at night and predation cues on foraging and predator avoidance in the keystone inshore mollusc Concholepas concholepas. Environ. Pollut., 280, 116895. https://doi.org/10.1016/j.envpol.2021.116895
Morin, J. G. (1983). Coastal bioluminescence: patterns and functions. Bull. Mar. Sci., 33(4), 787-817.
Nemade, L. P. (2023). Review on Thermal Analysis of LED Heat Power Dissipation and Efficiency Analysis. Int. J. Res. Appl. Sci. Eng. Technol., 11(11), 1284-1287. https://doi.org/10.22214/ijraset.2023.56685
Neun, S., Hintz, N. H., Schröder, M. & Striebel, M. (2022). Phytoplankton Response to Different Light Colors and Fluctuation Frequencies. Front. Mar. Sci., 9, 824624. https://doi.org/10.3389/fmars.2022.824624
Owens, A. C. S. & Lewis, S. M. (2021). Effects of artificial light on growth, development, and dispersal of two North American fireflies (Coleoptera: Lampyridae). J. Insect Physiol., 130, 104200. https://doi.org/10.1016/j.jinsphys.2021.104200
Owens, A. C. S., Van der Broeck, M., De Cock, R. & Lewis, S. M. (2022). Behavioral responses of bioluminescent fireflies to artificial light at night. Front. Ecol. Evol., 10, 946640. https://doi.org/10.3389/fevo.2022.946640
Park, S. A., Jeong, H. J., Ok, J. H., Kang, H. C., You, J. H., Eom, S. H., ... & Lee, M. J. (2024). Effect of salinity on the bioluminescence intensity of the heterotrophic dinoflagellates Noctiluca scintillans and Polykrikos kofoidii and the autotrophic dinoflagellate Alexandrium mediterraneum. Mar. Biol., 171, 126. https://doi.org/10.1007/s00227-024-04440-3
Price, J. T., Drye, B., Domangue, R. J. & Paladino, F. V. (2018). Exploring the role of artificial lighting in loggerhead turtle (Caretta caretta) nest-site selection and hatchling disorientation. Herpetol. Conserv. Biol., 13(2), 415-422.
Rabha, M. M., Sharma, U. & Barua, A. G. (2021). Light from a firefly at temperatures considerably higher and lower than normal. Sci. Rep., 11, 12498. https://doi.org/10.1038/s41598-021-91839-3
Ritchie, R. J. & Sma-Air, S. (2023). Microalgae grown under different light sources. J. Appl. Phycol., 35(2), 1-16. https://doi.org/10.1007/s10811-023-02917-0
Rodríguez, A., Dann, P. & Chiaradia, A. (2017). Reducing light-induced mortality of seabirds: High pressure sodium lights decrease the fatal attraction of shearwaters. J. Nat. Conserv., 39, 68-72. https://doi.org/10.1016/j.jnc.2017.07.001
Roenneberg, T. & Morse, D. (1993). Two circadian oscillators in one cell. Nature, 362(6418), 362-364. https://doi.org/10.1038/362362a0
Sathish, K., Saraswat, S. & Anusha, B. S. (2023). Light pollution and the impacts on biodiversity: the dark side of light. Biodiversity, 24(4), 194-199. https://doi.org/10.1080/14888386.2023.2244920
Satthong, S., Saego, K., Kitrungloadjanaporn, P., Nuttavut, N., Amornsamankul, S. & Triampo, W. (2019). Modeling the effects of light sources on the growth of algae. Adv. Differ. Equ., 170(1). 1-6. https://doi.org/10.1186/s13662-019-2112-6
Sherr, E. B. & Sherr B. F. (2007). Heterotrophic dinoflagellates: a significant component of microzooplankton biomass and major grazers of diatoms in the sea. Mar. Ecol. Prog. Ser., 352, 187-197. https://doi.org/10.3354/meps07161
Stomp, M., van Dijk, M. A., van Overzee, H. M. J., Wortel, M. T., Sigon, C. A. M., Egas, M., … & Huisman, J. (2008). The timescale of phenotypic plasticity and its impact on competition in fluctuating environments. Am. Nat., 172(8), 169-185. https://doi.org/10.1086/591680
Swift, E. & Meunier, V. (1976). Effects of light intensity on division rate, stimulable bioluminescence and cell size of the oceanic dinoflagellates Dissodinium lunula, Pyrocystis fusiformis and P. noctiluca. J. Phycol., 12(1), 14-22. https://doi.org/10.1111/j.1529-8817.1976.tb02819.x
Talanda, J., Maszczyk, P., Babkiewicz, E., Rutkowska, K. & Ślusarczyk, M. (2022). The short-term effects of planktivorous fish foraging in the presence of artificial light at night on lake zooplankton. J. Plankton Res., 44(6), 942-946. https://doi.org/10.1093/plankt/fbac046
Valiadi, M. & Iglesias-Rodríguez, D. (2013). Understanding Bioluminescence in Dinoflagellates – How Far Have We Come? Microorganisms, 1(1), 3-25. https://doi.org/10.3390/microorganisms1010003
Vitaterna, M. H., Takahashi, J. S. & Turek, F. W. (2001). Overview of Circadian Rhythms. Alcohol Res. Health, 25(2), 85-93.
Wang, L. (2018). Microbial control of the carbon cycle in the ocean. Natl. Sci. Rev., 5(2), 287-291. https://doi.org/10.1093/nsr/nwy023
Watanabe, Y. & Tanaka, Y. (2011). Bioluminescence-based imaging technique for pressure measurement in water. Exp. Fluids, 51, 225-236. https://doi.org/10.1007/s00348-011-1043-0
Zou, S. J., Shen, Y., Xie, F. M., Chen, J., Li, Y. & Tang, J. (2020). Recent Advances in Organic Light-Emitting Diodes: Toward Smart Lighting and Displays. Mater. Chem. Front., 4(3), 788-820. https://doi.org/10.1039/C9QM00716D
1 Department of Research Infrastructures for Marine Biological Resources, Stazione Zoologica Anton Dohrn, Via Po’ 25C, 00189, Rome (RM), Italy davide.dibari@szn.it* ORCID: https://orcid.org/0009-0003-6537-6092




