ISSN: 1659-455X • e-ISSN: 1659-407X
Vol. 17 (1), enero-junio 2025
Recepción 23 noviembre 2023 • Corregido 16 enero 2025 • Aceptado 16 enero 2025
DOI: https://dx.doi.org/10.15359/revmar.17-1.1
 |
First community level description of rocky reefs at the Cabo Blanco absolute natural reserve on Costa Rica’s Pacific Coast Primera descripción comunitaria de arrecifes Rocosos de la reserva natural absoluta Cabo Blanco en el pacífico de Costa Rica Andrés Jiménez-Solera1, Fausto Arias-Zumbado2, Andrea García-Rojas2, Yamileth Cubero-Campos1 & Karol Ulate-Naranjo*2 |
ABSTRACT
The Cabo Blanco Absolute Natural Reserve (RNACB) is located at the southern end of the Nicoya Peninsula in Costa Rica. This research aimed to provide the first community-level description of rocky reefs within RNACB. Data was collected in June 2018, covering a total area of 1680 m² monitored across six transects at two depths: -5 m and -20 m. A total of 44 macroinvertebrate species were recorded, primarily represented by suspension feeders and filter feeders, mainly including ascidians and octocorals, as well as 48 fish species, notably dominated by macroinvertivore species such as surgeonfish and parrotfish. The biomass recorded was 4.91 tons.ha-1, with the highest values predominantly observed at deeper sites. Sessile macroinvertebrates were predominantly found in deeper areas, while mobile organisms showed no specific depth preference. The average biomass of fish was greater in the deeper zones, and in general, RNACB biomass exceeded the expected values for the Costa Rican Pacific.
Keywords: Cabo Blanco Reserve, rocky reefs, community level description, biomass, PRONAMEC
RESUMEN
La Reserva Natural Absoluta Cabo Blanco (RNACB) se localiza en el extremo sur de la Península de Nicoya, Costa Rica. El objetivo principal de esta investigación fue realizar la primera descripción comunitaria de los arrecifes rocosos dentro de la RNACB. Los datos se obtuvieron en junio del año 2018. 1 680 m² se monitorearon en seis transeptos a dos profundidades: -5 m) y -20 m; se registraron 44 especies de macroinvertebrados dominadas por suspensívoros y filtradores especialmente ascidias y octocorales; y 48 especies de peces dominado por especies macroinvertívoras como cirujanos y loros; la biomasa obtenida fue de 4.91 ton.ha -1. Principalmente, la mayor riqueza se presentó en los sitios más profundos. Los macroinvertebrados sésiles dominaron los sitios profundos, pero los organismos móviles no tuvieron preferencia. La biomasa de peces tuvo promedios más altos en las zonas profundas y en general la biomasa en RNACB está por encima de los valores esperados para el Pacífico costarricense.
Palabras clave: Reserva Cabo Blanco, arrecife rocoso, descripción comunitaria, biomasa, PRONAMEC
Marine Protected Areas (MPAs) are crucial to ensuring the environmental services on which millions of people around the world depend (FAO, 2012; Cabral et al. 2019). They are important spawning and nursery sites for numerous marine species, assisting in the recovery of commercially valuable populations that are seriously damaged by overfishing and pollution, and also protect the physical structure of habitats from damage caused by fishing gear and other anthropogenic and incidental impacts (FAO, 2012).
Rocky reefs are very diverse and productive marine environments and are present on coasts with geological formation processes of volcanic origin (Horn & Ferry-Graham, 2006). Both rocky and coral reefs are an important source of economic income for many countries due to their contribution to local economies through tourism. They also constitute barriers that protect coastal areas from the effects of hurricanes and storms, and serve as a refuge breeding area for many species of commercial importance, especially in their juvenile stages, which is critically important for the fishing sector (Ahmed et al. 2005; van Oppen & Gates, 2006; NOAA, 2016).
The Cabo Blanco Absolute Natural Reserve (RNACB) is located at the southern end of the Nicoya Peninsula, Costa Rica. It was established in 1963 as the country’s first Protected Wildlife Area (PWA) and the only such area assigned to the management category of an Absolute Nature Reserve; its marine protected area (MPA) was created in 1982, which was also the first of its kind in Costa Rica (ACT et al. 2009). This is the only marine protected area among all the PWAs in the country in which any type of tourist, recreational, or resource use is not allowed within the marine area (ACT et al. 2009). These restrictive measures are intended to maintain the ecological integrity of the ecosystems present at the site, making it particularly interesting for carrying out research that evaluates the state of conservation of these ecosystems (Alvarado et al. 2006; Wehrtmann & Cortés, 2009). Community-level ecological assessments allow for a broader assessment of the health status of marine ecosystems inside and outside protected areas, providing reliable information on the effects of protection of a reef from fishing pressure and temporal changes in the structure of the community being studied (Aburto-Oropeza et al. 2015).
In MPAs, these community-level studies generate a solid and standardized baseline that can be replicated over time. The results of such studies are critically important in evaluating the condition of an ecosystem and guiding its adequate management. They also allow the evaluation of ecological phenomena such as “Top-Down” control, where organisms at higher levels in the food chain exert pressure and population control on organisms at lower trophic levels, maintaining ecosystem balance (Ulate et al. 2018; Jenkinson et al. 2020).
The main objective of this research was to carry out the first community-level description of rocky reefs within the RNACB and describe the biological community associated with the ecosystem of the oldest marine protected area in Costa Rica. This will contribute to achieving sustainable development goal 14 of the United Nations in improving knowledge of Costa Rica’s marine ecosystems for their conservation and sustainable use.
The methodology proposed by SINAC (2016), with adjustments for rocky reefs, was used for the ecological monitoring of coral formations, incorporating methodologies for the evaluation of fish biomass, density and size of macroinvertebrates, and the calculation of coverage of colonial sessile macroinvertebrates (SINAC-UNA, 2021).
The sampling sites were chosen through a bathymetric assessment of the rocky reefs in the RNACB, to corroborate the depths needed for sampling during June 2018 one shallow dive (~5m deep) and a separate deep dive (~20m deep). Each sampling site had three teams of two divers each, one dedicated to fish monitoring and one to macroinvertebrates, plus a safety diver (Dive Guide). Three (3) fish transects (50m x 5m x 5m) and three (3) invertebrate transects (30m x 1m) were conducted at two (2) different depths for a total sampling area of 1,680 m².
Study area:
Two sites were sampled within the RNACB, one shallow (~5 meters deep) and one deep (~20 meters). These rocky reefs were selected due to the structural composition of the substrate, which corresponds to a porous stone of volcanic origin (Horn & Ferry-Graham, 2006); the sampled sites did not contain walls or underwater slopes with high slope indices because these variables modify the community structure of the rocky reefs due to the incidence of light and influence of currents (Horn & Ferry-Graham, 2006) (Fig. 1).
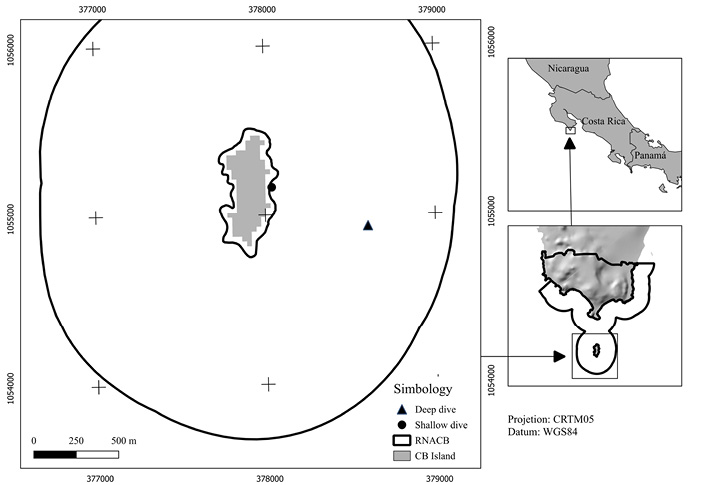
Fig. 1. Survey sites of the study area
Fig. 1. Sitios de muestreo en área de estudio
Vertebrates:
To quantify the abundance and diversity of fish in the reef, six transects of 50 m x 5 m each were established parallel to the coast, three at each depth, along an imaginary tunnel five meters wide (2.5 meters on each side of the transect) and five meters high; the individuals encountered were counted, and their sizes estimated, by species. Three transects were established at a depth of ~5 m (750 m2 total) and the other three at a depth of ~20 m (750 m2 total), for a total sampling area of 1 500 m2. Recorded fish were identified by in situ observations when possible and by using photographic records for subsequent identification at the species level with taxonomic guides (Allen & Ross-Robertson, 1998; Bussing & López, 2005; Humann & Deloach, 2004). Their abundance was counted, and their lengths were estimated using PVC tubes with alphanumeric scales that were graduated in 5 cm units. These measurements were made in situ without touching the individuals, using visual estimates.
The divers made runs along the transect at a constant speed without stopping, noting first the species in the water column, and upon returning focused on the observation of cryptic or hidden species in the substrate to improve the representativeness of the organisms found according to their distribution in the water column and the substrate.
Subsequently, the trophic levels of each of the identified fish were determined according to the following definitions (Froese & Pauly, 2014): Piscivores: Fish that feed mainly on other fish; Macro invertivores: Fish that feed mainly on macroinvertebrates; Herbivores: Fish that feed on algae; and Planktivores: Fish that feed on zooplankton.
As a population parameter, all abundance and size data for fish species were used to calculate biomass using the allometric length-weight conversion W=a*(Lt)b, where the parameters a and b are constant and specific for each species, Lt is the total length in cm, and W is the weight in grams. The length-weight parameters for each fish species were obtained from FishBase (Froese & Pauly, 2014).
Macroinvertebrates:
In the case of macroinvertebrates, the same six fish transects were used (the site locations and sampling depths remained the same – three at ~5m and three at ~20m), but with differences in the size of the sampling area, each of which was 30 m x 1 m, for a total sampling area of 180 m². All macroinvertebrates present within 50 cm on both sides of the transect were counted and measured.
All epibenthic macroinvertebrates larger than 1 cm (>1 cm) were identified in situ at the species level, when possible. Their abundance abundance was counted and their length or largest diameter was measured using PVC tubes with 2 cm scales, without touching individuals. Organisms that could not be identified at the species level were also considered as operant taxonomic units (OTU), and their identification was carried out using photographs. The censused organisms were not extracted or manipulated, focusing only on the epibenthos, and preserving the marine landscape, while an attempt was made to take as many photographs as possible for later identification (Behrens & Hermosillo 2005; Bertsch & Kerstitch 2007; Gotshall 1998; Humann & Deloach 2012).
Subsequently, the trophic levels of the observed macroinvertebrates were identified according to the following definitions (Ulate et al. 2016): Carnivores: Macroinvertebrates whose primary consumption is the meat of fish or other macroinvertebrates, either caught directly or available due to other circumstances; Herbivores: Macroinvertebrates whose diet is based on macroalgae; Filter feeders: Macroinvertebrates that obtain their food (plankton or organic particles) using water pumping and filtration systems within their bodies; Suspensivores: Macroinvertebrates that obtain their food by capturing plankton and/or organic particles suspended in the water column; and Hetero/Autotrophs: Macroinvertebrates that alternate their diets, consuming either by products derived from photosymbionts (e.g., zooxanthellae that live in corals), or organisms or food particles that they can capture for themselves.
The density of organisms per square meter (org.m²) was used as a population parameter to report unitary or non-colonial mobile and sessile macroinvertebrates.
Coverage of colonial sessile macroinvertebrates
To define the average cover of colonies of colonial sessile macroinvertebrates such as colonial hydrozoans and colonial ascidians, including zooxanthellate scleractinian corals (stony corals), the same transects of macroinvertebrates were used following the method of Chiappone & Sullivan (1991), determining the diameter of the macroinvertebrate colony using the formula for the area of a circle (area = π*r2). Thus, based on the reported size (diameter) for each colony, the area (m2) occupied by the species in each transect was estimated (Chiappone & Sullivan, 1991).
Descriptive analysis of the fauna:
To determine the ecological dominance of the species encountered, population parameters were derived using densities (org.m2) in the case of macroinvertebrates and biomass (ton.ha-1) in the case of fish, concerning the percentage of occurrence in each site at each sampling depth. Groups of dominant species were then estimated using the Olmstead-Tukey method (Rohlf & Sokal, 1981), based on graphs of the average densities or biomasses of all species and/or OTUs (X axis), against the percentage of occurrence of appearance in the transects of the species and/or OTUs (Y axis). This technique allows establishing a classification according to values of means density or biomass and frequency of concurrence of the organisms, following García de León (1988):
• Dominant: Those species whose values for both density or biomass and frequency of occurrence exceed the arithmetic mean of these estimators.
• Frequent: Those species whose values for frequency of occurrence are higher than the average for this estimator but whose values for relative density or biomass are not higher than the average value of these estimators
• Occasional: Those species whose density or biomass values are higher than the average for these estimators but whose values for frequency of occurrence are not higher than the average value of this estimator.
• Rare: Those species that are characterized by values for both density or biomass and frequency of occurrence below the arithmetic mean for these estimators.
We determined that the survey sites with a highly complex and irregular substrate with a horizontal orientation, due to their morphological characteristics and rock composition of volcanic origin, with incrustations of living organisms, correspond to a rocky reef.
In the study area, 1 680 m2 were monitored, and data on a total of 3 204 were recorded, of which 2047 individuals were macroinvertebrates while 1157 individuals were fish.
Macroinvertebrate richness
The biodiversity of macroinvertebrates in the RNACB was distributed as follows: 7 phyla, 16 families, 44 species and 2047 individuals (Annex 1).
When comparing the sampled sites, the average values of macroinvertebrate species richness are highest for deep zones in the RNACB (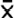 = 19.33 ± 8.57), where groups of filter feeders (mainly ascidians) and suspension feeders (octocorals) contain the most species, while in the shallow zones the average richness was
= 19.33 ± 8.57), where groups of filter feeders (mainly ascidians) and suspension feeders (octocorals) contain the most species, while in the shallow zones the average richness was 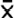 = 15.33 ± 3.46 (Fig. 2).
= 15.33 ± 3.46 (Fig. 2).
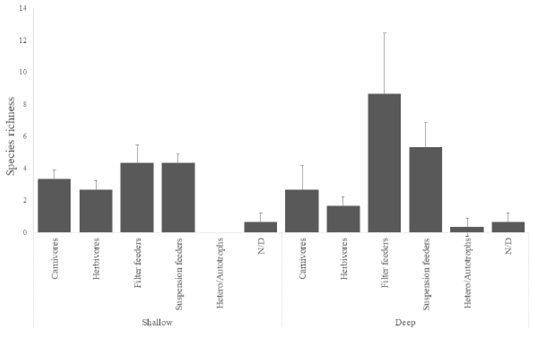
Fig. 2. Average macroinvertebrate species richness in the rocky reefs of the RNACB. N/D Not identified organisms
Fig. 2. Promedio de riqueza de especies de macroinvertebrados en los arrecifes rocosos de la RNACB. N/D organismos no identificados
Vertebrates richness
The biodiversity of fish in the RNACB was distributed as follows: 17 families, 48 species and 1157 individuals (Annex 2). The average values of fish species richness were lowest in shallow zones in the RNACB (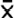 = 12.66 ± 11.24). The group with the highest number of fish species was macro invertivores, while in the deep zones the average richness was
= 12.66 ± 11.24). The group with the highest number of fish species was macro invertivores, while in the deep zones the average richness was 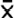 = 19 ± 6.74 (Fig. 3).
= 19 ± 6.74 (Fig. 3).
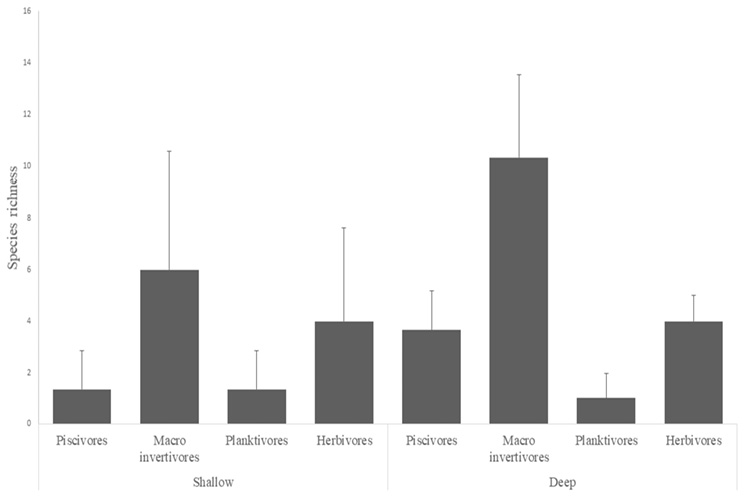
Fig. 3. Average vertebrate species richness in the rocky reefs of the RNACB
Fig. 3. Promedio de riqueza de especies de vertebrados en los arrecifes rocosos de la RNACB
Average coverage of colonial sessile macroinvertebrates
According to the values obtained for average coverage or area of the size of colonies of colonial macroinvertebrates (deep zones 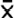 = 5.81m2 ± 4.78 and shallow zones
= 5.81m2 ± 4.78 and shallow zones 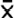 = 1.25m2 ± 1.30), suspension feeders such as the snowflake octocoral (Carijoa riisei), and two species of gorgonians (Leptogorgia rigida and Leptogorgia alba) have the largest average areas (
= 1.25m2 ± 1.30), suspension feeders such as the snowflake octocoral (Carijoa riisei), and two species of gorgonians (Leptogorgia rigida and Leptogorgia alba) have the largest average areas (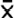 = 4.92m2 ± 3.94 in the deep zones). Furthermore, it is important to note that the coverage of rocky coral colonies (Hetero/Autrophs) was very low in all sampling sites
= 4.92m2 ± 3.94 in the deep zones). Furthermore, it is important to note that the coverage of rocky coral colonies (Hetero/Autrophs) was very low in all sampling sites 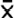 = 0.03m2 ± 0.05 (Fig. 4).
= 0.03m2 ± 0.05 (Fig. 4).
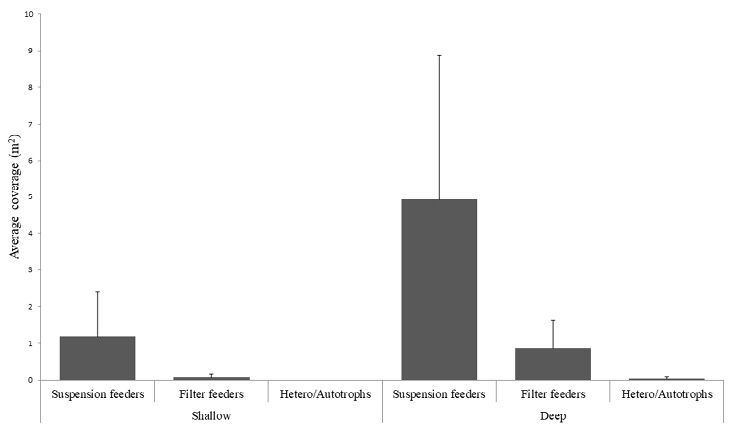
Fig. 4. Average coverage of macroinvertebrates in the rocky reefs of the RNACB
Fig. 4. Cobertura promedio de macroinvertebrados en los arrecifes rocosos de la RNACB
Average macroinvertebrate density
Average density values for non-colonial macroinvertebrates show that filter feeders such as the blue sea squirt (Rhopalaea birkelandi), the mother-of-pearl bivalve (Pinctada mazatlanica), and some species of oysters that are difficult to identify in situ (Ostrea sp) had the highest density per square meter (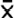 = 1.37org.m2 ± 0.82 in deep zones and
= 1.37org.m2 ± 0.82 in deep zones and 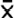 = 0.78org.m2 ± 0.38 in shallow zones). Likewise, the trophic level of suspension-feeding organisms such as the polychaetes called Christmas trees of the genus Spirobranchius, the polychaetes known as spaghetti worms of the family Terebellidae, and suspension-feeding sea cucumbers of the genus Neothyonidium sp, were the species with the highest density (
= 0.78org.m2 ± 0.38 in shallow zones). Likewise, the trophic level of suspension-feeding organisms such as the polychaetes called Christmas trees of the genus Spirobranchius, the polychaetes known as spaghetti worms of the family Terebellidae, and suspension-feeding sea cucumbers of the genus Neothyonidium sp, were the species with the highest density (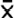 = 0.11org.m2 ± 0.17 in deep zones and
= 0.11org.m2 ± 0.17 in deep zones and 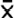 = 1.38org.m2 ± 0.36 in shallow zones). In general, deep RNACB reefs had an average macroinvertebrate density of 1.81 org.m2 ± 1.31 (Fig. 5).
= 1.38org.m2 ± 0.36 in shallow zones). In general, deep RNACB reefs had an average macroinvertebrate density of 1.81 org.m2 ± 1.31 (Fig. 5).
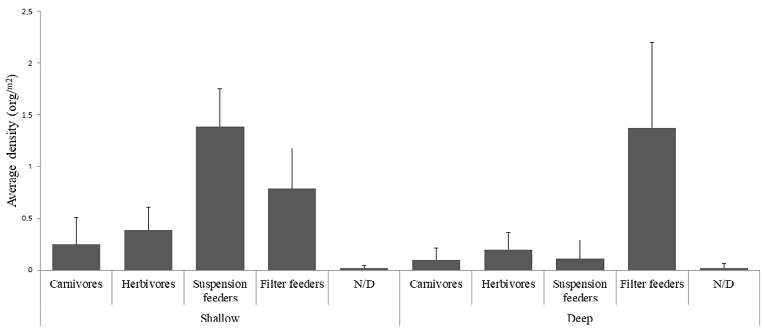
Fig. 5. Average density of macroinvertebrates in the rocky reefs of the RNACB. N/D means unidentified organisms
Fig. 5. Densidad promedio de macroinvertebrados en los arrecifes rocosos de la RNACB. N/D organismos no identificados
Average Vertebrates biomass
The biomass values show significant differences between strata and sizes of organisms. Figure 6 shows that the deep zones had higher average biomass values (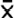 = 8.26 ± 8.34), while at the shallow zones the average biomass was
= 8.26 ± 8.34), while at the shallow zones the average biomass was 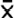 =1.55 ± 1.38; the sum of average biomasses at each trophic level indicates that the RNACB has a total average biomass of 4.91 ± 4.86 ton.ha-1.
=1.55 ± 1.38; the sum of average biomasses at each trophic level indicates that the RNACB has a total average biomass of 4.91 ± 4.86 ton.ha-1.
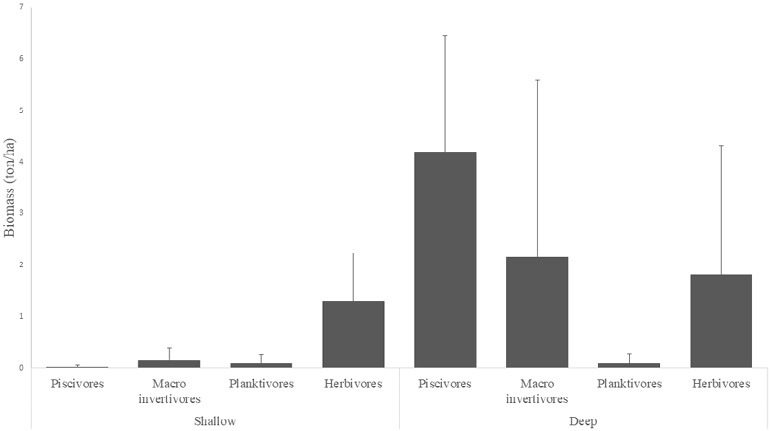
Fig. 6. Vertebrate biomass in the rocky reefs of the RNACB
Fig. 6. Biomasa de vertebrados en los arrecifes rocosos de la RNACB
Macroinvertebrate dominance analysis
Dominance analyses were used to describe the composition of the species at each sampling site. Analysis of dominance by depth showed that two species were dominant in both deep and shallow zones – the blue sea squirt (R. birkelandi) and the gorgonian Leptogorgia alba. Other species such as the sea urchin (Diadema mexicanum) and the pencil urchin (Eucidaris thouarsii) were dominant mainly in the shallow sites, although they were also present in the deep sites. The gorgonian known as snowflake coral (Carijoa riisei) was dominant mainly in deep sites, with very little presence in the shallow environments sampled (Fig. 7 and Fig. 8). These species are also classified as dominant for RNACB in the results of analysis of pooled data for both deep and shallow sites (Fig. 9).
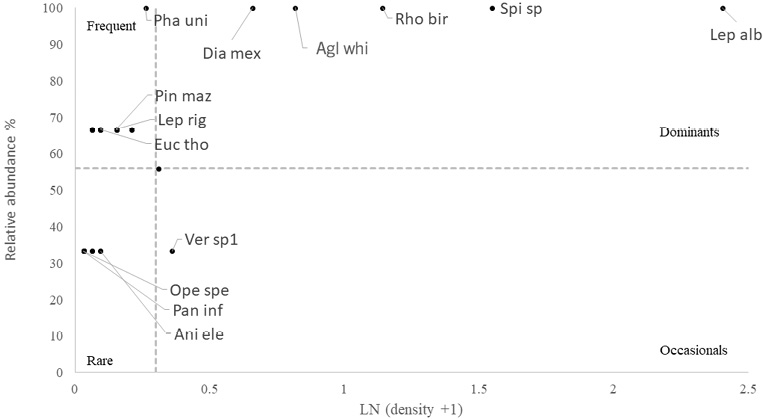
Fig. 7. Dominance of macroinvertebrates in the shallow zones of rocky reefs of the RNACB (species acronyms include the first three letters of the genus and the first three letters of the species)
Fig. 7. Dominancia de macroinvertebrados en las zonas someras de los arrecifes rocosos de la RNACB (los acrónimos de las especies incluyen las tres primeras letras del género y las tres primeras letras de la especie)
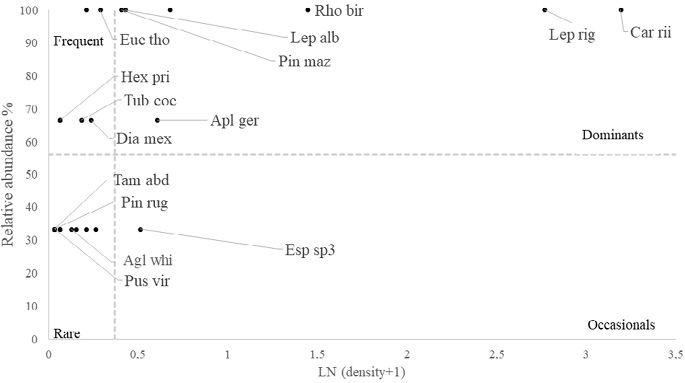
Fig. 8. Dominance of macroinvertebrates in the deep zones of rocky reefs of the RNACB (species acronyms include the first three letters of the genus and the first three letters of the species)
Fig. 8. Dominancia de macroinvertebrados en las zonas profundas en los arrecifes rocosos de la RNACB (Los acrónimos de las especies incluyen las tres primeras letras del género y las tres primeras letras de la especie)
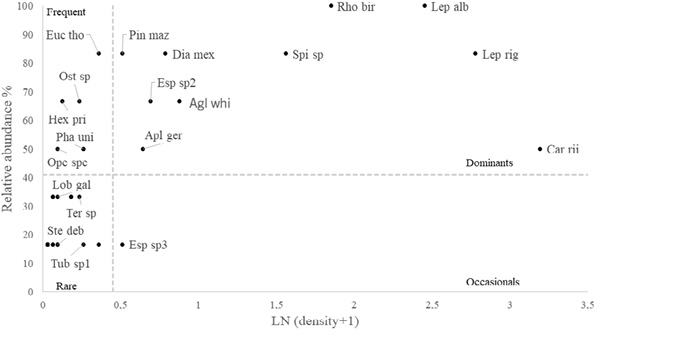
Fig. 9. Dominance of macroinvertebrates at all depths in the rocky reefs of the RNACB (Species acronyms include the first three letters of the genus and the first three letters of the species)
Fig. 9. Dominancia de macroinvertebrados en todas las profundidades en los arrecifes rocosos de la RNACB. (los acrónimos de las especies incluyen las tres primeras letras del género y las tres primeras letras de la especie)
Fish dominance analysis
In the shallow RNACB, only the razor surgeonfish (Prionurus laticlavius) was recorded as dominant (Fig. 10), while in the deep zone, the dominant species were herbivorous and macroinvertivorous species such as the razor surgeonfish (Prionurus laticlavius) and the blacknosed butterflyfish (Johnrandallia nigrirostris) (Fig. 11). The piscivorous species known as the bigeye jack (Caranx sexfasciatus), in particular, was recorded as occasional in the deep RNACB, being very abundant but found in only a few transects; however, in the analysis of the ecosystem as a whole (using pooled data from shallow and deep strata sites) for the RNACB, this species was classified as dominant (Fig. 12).
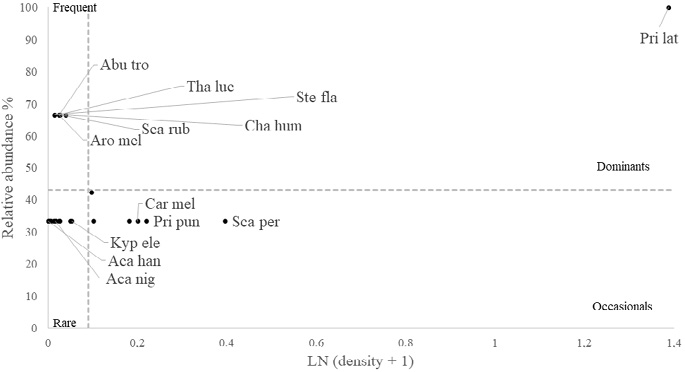
Fig. 10. Dominance of vertebrates in the shallow zones of rocky reefs of the RNACB (species acronyms include the first three letters of the genus and the first three letters of the species)
Fig. 10. Dominancia de vertebrados en las zonas someras los arrecifes rocosos de la RNACB (los acrónimos de las especies incluyen las tres primeras letras del género y las tres primeras letras de la especie)

Fig. 11. Dominance of vertebrates in the deep zones of rocky reefs of the RNACB (species acronyms include the first three letters of the genus and the first three letters of the species)
Fig. 11. Dominancia de vertebrados en las zonas profundas en los arrecifes rocosos de la RNACB (los acrónimos de las especies incluyen las tres primeras letras del género y las tres primeras letras de la especie)
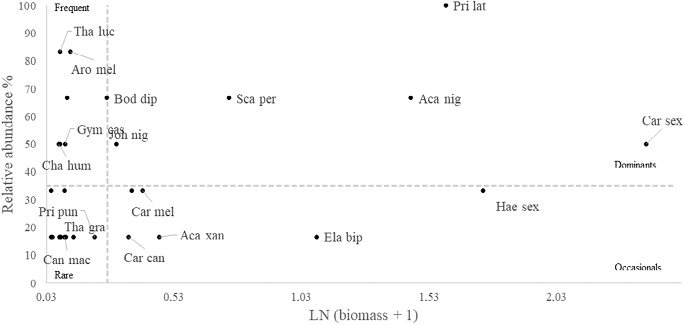
Fig. 12. Dominance of vertebrates at all depths in the rocky reefs of the RNACB (species acronyms include the first three letters of the genus and the first three letters of the species)
Fig.12. Dominancia de vertebrados en todas las profundidades en los arrecifes rocosos de la RNACB (los acrónimos de las especies incluyen las tres primeras letras del género y las tres primeras letras de la especie)
The rocky reefs of the RNACB are characterized by a reduced and very patchy distribution of corals throughout the reef area (BIOMARCC-SINAC-GIZ, 2013). Corals are therefore not dominant, and their function as providers of shelter and reproductive areas is assumed by irregular structures and cavities within the rock formations, mostly of volcanic origin, inhabited by macroalgae and a diversity of macroinvertebrates such as octocorals, sponges, ascidians, crustaceans and mollusks, in addition to fish that forage on these organisms (Robertson & Allen, 2015).
This situation was evident in all the sites studied in this investigation, which encountered a great diversity and abundance of organisms, mainly in the groups of invertebrate suspension feeders (Octocorallia) and filter feeders (Demospongiae and Ascidiacea), as well as fish, which were also highly diverse, particularly piscivores, macro invertivores and herbivores, which had the highest values for species richness.
This richness and abundance of species belonging to the groups of filter feeders and suspension feeders in all monitoring sites may be associated with the fact that these sites are high energy areas exposed to currents resulting from waves and changes in tides (Lizano & Alfaro, 2014), which could facilitate obtaining food carried in the water. However, more information about this topic is needed to reliably relate oceanic conditions of a particular site to the establishment of suspension-feeding and filter-feeding organisms.
Herbivorous organisms dependent on macroalgae, such as the urchins Eucidaris thouarsii and Diadema mexicanum, were recorded as frequent in the deep sites of the RNACB, probably as a result of higher competitive pressure in the shallow sites from fish of the same trophic level, or by an abundance of juvenile stages of predators present in the shallow areas. These species of urchins, being herbivorous, depend on the production of algae for their food; their presence indicates that there is adequate light penetration in the sites studied that provides the conditions for establishing both macroalgae and the organisms that feed on them. Diadema mexicanum is a widely distributed and abundant species in tropical reefs (Muthiga & Mcclanahan, 2007; Benitez Villalobos et al. 2008); and is considered to be an important bioeroder of reefs, with a great impact on the restructuring and modeling of these ecosystems (Arias-Godínez et al. 2019; Glynn et al. 2020); therefore, constant monitoring of this organism is essential for ecosystem evaluation. Additionally, the presence and sizes of these organisms are an indirect indication of the absence of predatory fish at the macroinvertivorous trophic level, demonstrating an imbalance in the trophic chain and therefore inefficient management of the protected area.
Other sessile invertebrates that were an important component in the sampling sites were the octocorals Leptogorgia alba and Carijoa riisei, whose dominance in the ecosystem analysis results is consistent with comments by Liberman et al. (2018) and Romero-Hernández (2018) about the importance of light for the establishment of corals and octocorals. Leptogorgia alba is a common and dominant species in the rocky reefs of the Pacific of Costa Rica and particularly in the RNACB (Breedy & Cortés, 2014) but uncommon in others in the Pacific such as the Malpelo Islands of Colombia (Sánchez et al. 2016). For its part, Carijoa riisei is a widely distributed species and considered an invasive pest in South American reefs (Sánchez et al. 2016; Sánchez & Ballesteros, 2014). The percentage of coverage of this species was found to be substantial in this study; however, until its coverage is evaluated over time, its presence cannot yet reliably be considered a problem, requiring recommendations for its management.
Another species that is not as dependent on light but was dominant in all sites at both depths was the blue sea squirt (R. birkelandi). This species is considered to be common and dominant in studies carried out on rocky reefs in the Panamanian Pacific (Bullard et al. 2011) and Costa Rica (Nova-Bustos et al. 2010), in addition to being very important in the diet of chelonians, especially the Hawksbill turtle (Eretmochelys imbricata) in the northern Pacific of the country (Carrión-Cortez et al. 2013).
In the case of fish, the dominant species in deep sites are all herbivores. There was no dominance of top species, since large schools of the browsing herbivorous species White-cheeked Surgeonfish (Acanthurus nigricans) and the razor surgeonfish (Prionurus laticlavius) dominated reefs (Carr et al. 2018; Zgliczynski et al. 2019), together with the bumphead parrotfish (Scarus perrico), a herbivorous species (Melgarejo-Damián et al. 2018), of interest due to the pressure it has been suffering from human consumption and its importance in reef communities in grazing algae, and as builders of fine reef sediments (Robertson & Allen, 2015; Ross et al. 2017). Similarly, in the shallow sites, the razor surgeonfish (Prionurus laticlavius) was the only species considered to be dominant; as an herbivore (Carr et al. 2018), its presence is expected in sites with adequate light penetration.
A species that stood out in the monitoring was the bigeye jack (Caranx sexfaciatus), since large schools of individuals of this species were occasionally observed, causing biomass to increase sharply in the deep RNACB. This species has been used in the Philippines as an indicator of good health in marine protected areas due to its importance for recreational tourism and fishing, and for being a top species in food chains (Maypa, 2012); and the large biomass levels of this species are associated with unfished or ‘pristine’ areas (Barrett et al. 2019). In Costa Rica, it is commercially important in artisanal and sport fishing (Ross et al. 2017), and the presence of this species in the RNACB can therefore be regarded as an indicator of adequate management of the marine protected area.
In areas with strict protection such as the RNACB, it is reasonable to expect to find healthier ecosystems in nearly optimal conditions which could function as a reference point for other sites (Brander et al. 2020). It has been shown that biomass of all fish assemblages increases significantly in areas with absolute, and even partial, protection (Beita-Jiménez et al. 2019; Sala & Giakoumi, 2018). Using statistical methods, McClanahan et al. (2007) and McClanahan & Graham (2015) determined that, in unfished areas within national parks in the Indian Ocean with more than 12 years of protection, a healthy biomass of fish larger than 10 cm should be between 1.1 and 1.2 ton.ha-1. In the Cabo Pulmo National Park in Mexico, Aburto-Oropeza et al. (2015) estimated that fish biomass had increased by 3.4 ton.ha-1. The results obtained for the RNACB (4.91 ton.ha-1) are higher than these figures, although lower than biomass estimates of 12.5 ton.ha-1 for the Cocos Island National Park in the Pacific of Costa Rica (Fourriére et al. 2019), but the data obtained in this study should be interpreted with caution. It must be taken into account that the Costa Rican Pacific Coast has been subject to fishing for many years, so biomass values greater than 1.5 ton.ha-1 are considered normal for the region (Alvarado et al. 2018; Cordero-Umaña & Santidrián-Tomillo, 2020).
The Cabo Blanco Absolute Nature Reserve is a protected marine area, made up in its marine sector mainly of rocky reefs with the presence of small patches of rocky coral, with octocorals and sponges predominating in the rock formations of the reef.
The richness of invertebrates was greatest in deep sites, especially species of sponges and octocorals.
Fish richness was similar at both shallow and deep zones, with herbivores dominating. The greatest richness of piscivores was recorded in deep sites.
The sessile macroinvertebrates that dominated the deep sites were mostly suspension feeders such as octocorals and filter feeders such as ascidians.
The density of mobile organisms was higher for the filter-feeding and suspension-feeding groups at both depths of the RNACB.
The average biomass of fish was higher in the deep zones, and in general the biomass in RNACB is above the expected values for the Costa Rican Pacific.
The snowflake octocoral (Carijoa riisei) was found to have a high coverage that could be considered a demographic explosion. There are reports in the Panamanian and Ecuadorian Pacific indicating that it can negatively affect the reefs in which it establishes itself.
The low abundances and large sizes of sea urchin populations of the species Diadema mexicanum and Eucidaris thouarsii, as well as the high fish biomass values obtained in the RNACB could be considered to indicate that the reef of the Cabo Blanco Absolute Nature Reserve is in good condition compared to other sites on the northern Pacific Coast of Costa Rica.
The work was funded by the Special Fund for Higher Education (FEES) of the National University as part of the project called: “Health status of the rocky reef ecosystems of the marine protected areas in the Costa Rican North Pacific” of the School of Biological Sciences of the National University, SIA: 0592-16.
We also wish to recognize the assistance of the staff of the Cabo Blanco Absolute Nature Reserve, the National System of Conservation Areas, the Tempisque Conservation Area and the diving company Iguana Divers. And finally, we would like to thank reviewers for taking the time and effort necessary to evaluate the manuscript. We sincerely appreciate all valuable comments and suggestions, which helped us to improve the quality of the manuscript.
Aburto-Oropeza, O., Ezcurra, E., Moxley, J., Sánchez-Rodríguez, A., Mascareñas-Osorio, I., Sánchez-Ortiz, C., … & Ricketts, T. (2015). A framework to assess the health of rocky reefs linking geomorphology, community assemblage, and fish biomass. Ecol. Indic., 52, 353-361. https://doi.org/10.1016/j.ecolind.2014.12.006
ACT, SINAC & MINAET. (2009). Plan General de Manejo de la Reserva Natural Absoluta Cabo Blanco. Costa Rica: Sin Editorial.
Ahmed, M., Chong, C. K. & Cesar, H. (Eds.). (2005). Economic Valuation and Policy Priorities for Sustainable Management of Coral Reefs. Malasya. WorldFish Center.
Allen, G. R. & Ross-Robertson, D. (1998). Peces del Pacífico Oriental Tropical. 2da Ed. México: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad y Agrupación Sierra Madre, S.C.
Alvarado, J., Fernández, C. & Nielsen, V. (2006). Arrecifes y Comunidades Coralinas. En V. Nielsen Muñoz & M. A. Quesada Alpízar (Eds.), Ambientes Marino Costeros de costa Rica. Comisión Interdisciplinaria Marino Costera de la Zona Económica Exclusiva de Costa Rica. (pp. 51-68). Costa Rica: Conservación Internacional.
Alvarado, J. J., Beita-Jiménez, A., Mena, S., Fernández, C., Cortés, J., Sánchez-Noguera, C., … & Guzmán-Mora, A. G. (2018). Cuando la conservación no puede seguir el ritmo del desarrollo: Estado de salud de los ecosistemas coralinos del Pacífico Norte de Costa Rica. Rev. Biol. Trop., 66(S1), S280-S308. https://doi.org/10.15517/rbt.v66i1.33300
Arias-Godínez, G., Jiménez, C., Gamboa, C., Cortés, J., Espinoza, M. & Alvarado, J. J. (2019). Spatial and temporal changes in reef fish assemblages on disturbed coral reefs, north Pacific Coast of Costa Rica. Mar. Ecol., 40(1), e12532. https://doi.org/10.1111/maec.12532
Barrett, L. T., de Lima, A. & Goetze, J. S. (2019). Evidence of a biomass hotspot for targeted fish species within Namena Marine Reserve, Fiji. Pac. Conserv. Biol., 25(2), 204-207. https://doi.org/10.1071/PC18034
Behrens, D. W. & Hermosillo, A. (2005). Eastern Pacific Nudibranchs. A guide to the Opisthobranchs from Alaska to Central America. EE. UU.: Sea Challengers, Inc.
Beita-Jiménez, A., Alvarado, J. J., Mena, S. & Guzmán-Mora, A. G. (2019). Benefits of protection on reef fish assemblages in a human impacted region in Costa Rica. Ocean. Coast. Manag., 169, 165-170. https://doi.org/10.1016/j.ocecoaman.2018.12.023
Benitez Villalobos, F., Gómez, M. T. & López-Pérez, A. (2008). Temporal variation of the sea urchin (Diadema mexicanum) population density at Bahías de Huatulco, Western Mexico. Rev. Biol. Trop., 56(S3), 255-263. https://doi.org/10.15517/rbt.v56i3.27140
Bertsch, H. & Kerstitch, A. (2007). Sea of Cortez Marine Invertebrates: A Guide for the Pacific Coast, Mexico to Peru (2th Edition). EE. UU.: Sea Challengers, Inc.
BIOMARCC-SINAC-GIZ. (2013). Estudios científicos de hábitat marino costero y situación socioeconómica del Pacífico Sur de Costa Rica. Costa Rica: BIOMARCC.
Brander, L. M., van Beukering, P., Nijsten, L., McVittie, A., Baulcomb, C., Eppink, F. V. & Cado van der Lelij, J. A. C. (2020). The global costs and benefits of expanding Marine Protected Areas. Mar. Polic., 116, 103953. https://doi.org/10.1016/J.MARPOL.2020.103953
Breedy, O. & Cortés, J. (2014). Gorgonias (Octocorallia: Gorgoniidae) de las aguas someras del Pacífico Norte de Costa Rica. Rev. Biol. Trop., 62(4), 43-62. https://doi.org/10.15517/rbt.v62i4.20032
Bullard, S. G., Carman, M. R., Rocha, R. M., Dijkstra, J. A. & Goodin, A. M. (2011). Abundance and diversity of ascidians in the southern Gulf of Chiriquí, Pacific Panama. Aquatic. Inv., 6(4), 381-390. https://doi.org/10.3391/ai.2011.6.4.03
Bussing, W. A. & López, M. I. (2005). Peces de la Isla del Coco y Peces Arrecifales de la costa Pacífica de América Central Meridional. Rev. Biol. Trop., 53(2), 1-192.
Cabral, R. B., Halpern, B. S., Lester, S. E., White, C., Gaines, S. D. & Costello, C. (2019). Designing MPAs for food security in open-access fisheries. Sci. Rep., 9(1), 8033. https://doi.org/10.1038/s41598-019-44406-w
Carrión-Cortez, J., Canales-Cerro, C., Arauz, R. & Riosmena-Rodríguez, R. (2013). Habitat use and diet of juvenile eastern pacific hawksbill turtles (Eretmochelys imbricata) in the north Pacific Coast of Costa Rica. Chelonian Conserv. Biol., 12(2), 235-245. https://doi.org/10.2744/CCB-1024.1
Carr, L. A., Gittman, R. K. & Bruno, J. F. (2018). Temperature influences herbivory and algal biomass in the Galápagos Islands. Front. Mar. Sci., 5, 1-10. https://doi.org/10.3389/fmars.2018.00279
Chiappone, M. & Sullivan, K. M. (1991). A comparison of line transect versus linear percentage sampling for evaluating stony coral (Scleractinia and Milleporina) community similarity and area coverage on reefs of the central Bahamas. Coral Reefs, 10, 139-154. https://doi.org/10.1007/BF00572173
Cordero-Umaña, K. E. & Santidrián-Tomillo, P. (2020). Conservation status of fish and marine invertebrate of rocky reefs and sandy substrates in two unprotected bays of the papagayo gulf, Costa Rica. Rev. Biol. Trop., 68(4), 1311-1321. https://doi.org/10.15517/RBT.V68I4.42007
FAO. (2012). Estado de las Áreas Marinas y Costeras Protegidas en América Latina. Chile. REDPARQUES Cuba.
Fourriére, M., Alvarado, J. J., Cortés, J., Taylor, M. H., Ayala-Bocos, A., Azofeifa-Solano, J. C., … & Wolff, M. (2019). Energy flow structure and role of keystone groups in shallow water environments in Isla del Coco, Costa Rica, Eastern Tropical Pacific. Ecol. Model., 396, 74-85. https://doi.org/10.1016/j.ecolmodel.2019.01.004
Froese, R. & Pauly, D. (2014). FishBase. World Wide Web electronic publication. http://www.fishbase.org
García de León, A. (1988). Generalidades del análisis de cúmulos y del análisis de componentes principales (8th ed.). México: Instituto de Geografía, Universidad Nacional Autónoma de México.
Glynn, P. J., Glynn, P. W., Maté, J. & Riegl, B. (2020). Agent-based model of Eastern Pacific damselfish and sea urchin interactions shows increased coral reef erosion under post-ENSO conditions. Ecol. Model., 423, 108999. https://doi.org/https://doi.org/10.1016/j.ecolmodel.2020.108999
Gotshall, D. W. (1998). Sea of Cortez marine animals: A guide to the common fishes and invertebrates: Baja California to Panama. EE. UU.: Sea Challengers, Inc.
Horn, M. H. & Ferry-Graham, L. A. (2006). Feeding Mechanisms and Trophic Interactions. In L. G. Allen, D. J. Pondella II & B. M. H. Horn (Eds.), The Ecology of Marine Fishes (pp. 387-410). EE.UU.: University of California Press.
Humann, P. & Deloach, N. (2004). Reef Fish Identification: Baja to Panama. EE. UU.: New World Publications, Inc.
Humann, P. & Deloach, N. (2012). Reef Creature Identification: Tropical Pacific. EE. UU.: New World Publications, Inc.
Jenkinson, R. S., Hovel, K. A., Dunn, R. P. & Edwards, M. S. (2020). Biogeographical variation in the distribution, abundance, and interactions among key species on rocky reefs of the northeast Pacific. Mar. Ecol. Progr. Ser., 648, 51-65. https://doi.org/10.3354/meps13437
Liberman, R., Shlesinger, T., Loya, Y. & Benayahu, Y. (2018). Octocoral sexual reproduction: Temporal disparity between mesophotic and shallow-reef populations. Front. Mar. Sci., 5, 445. 1-14. https://doi.org/10.3389/fmars.2018.00445
Lizano R., O. G. & Alfaro M., E. J. (2014). Dinámica atmosférica y oceánica en algunos sitios del Área de Conservación Guanacaste (ACG), Costa Rica. Rev. Biol. Trop., 62(S4), 17-31. https://doi.org/10.15517/rbt.v62i4.20018
Maypa, A. (2012). Mechanisms by which marine protected areas enhance fisheries benefits in neighboring areas. (Unpublished Doctoral thesis). University of Hawaii, Manoa.
McClanahan, T. R. & Graham, N. A. J. (2015). Marine reserve recovery rates towards a baseline are slower for reef fish community life histories than biomass. Proc. R. Soc. B. Biol. Sci., 282(1821), 20151938. 1-10.-https://doi.org/10.1098/rspb.2015.1938
McClanahan, T. R., Graham, N. A. J., Calnan, J. M. & MacNeil, M. A. (2007). Toward pristine biomass: Reef fish recovery in coral reef marine protected areas in Kenya. Ecol. Applic., 17(4), 1055–1067. https://doi.org/10.1890/06-1450
Melgarejo-Damián, M. P., González-Acosta, A. F., Cruz-Escalona, V. H. & Moncayo-Estrada, R. (2018). A comparison of feeding biomechanics between two parrotfish species from the Gulf of California. Zoomorphology, 137(1), 165-176. https://doi.org/10.1007/s00435-017-0383-6
Muthiga, N. A. & McClanahan, T. R. (2007). Ecology of Diadema. In J. M. Lawrence (Ed.), Edible sea urchins: biology and ecology (pp. 205-225). Netherlands: Elsevier.
NOAA. (2016). Rocky Reef on the West Coast. National Oceanic and Atmospheric Administration. https://www.fisheries.noaa.gov/west-coast/habitat-conservation/rocky-reef-west-coast
Nova-Bustos, N., Hernández-Zanuy, A. C. & Viquez-Portuguez, R. (2010). Distribución y abundancia de las ascidias de los fondos rocosos de la Bahía de Cuajiniquil, Costa Rica. Bol. Investig. Mar. Cost. - INVEMAR, 39, 57-66.
Robertson, D. & Allen, G. (2015). Peces Costeros del Pacífico Oriental, Sistema de Información en línea. https://biogeodb.stri.si.edu/sftep/es/pages/generalinfo#6.1
Romero-Hernández, Y. (2018). Efecto de la sedimentación en el desarrollo de los arrecifes coralinos. Rev. Investig. Agropec. Des. Sost., 3(2), 42-49.
Ross, E., Posada, J., Melo, G., Suárez, C., Rojas-Ortega, G. & Ventura, A. (2017). Guía de identificación: peces de importancia comercial en la costa pacífica de Costa Rica. Costa Rica: Fundación MarViva.
Sala, E. & Giakoumi, S. (2018). No-take marine reserves are the most effective protected areas in the ocean. ICES J. Mar. Sci., 75(3), 1166-1168. https://doi.org/10.1093/icesjms/fsx059
Sánchez, J. A. & Ballesteros, D. (2014). The invasive snowflake coral (Carijoa riisei) in the tropical Eastern Pacific, Colombia. Rev. Biol. Trop., 62, 199-207. https://doi.org/10.15517/rbt.v62i0.16276
Sánchez, J. A., Gómez, C. E., Escobar, D. & Dueñas, L. F. (2016). Diversidad, Abundancia y Amenazas de los Octocorales de la Isla Malpelo, Pacífico Oriental Tropical, Colombia. Bull. Mar. Coast. Res., 40, 139-154. https://doi.org/10.25268/bimc.invemar.2011.40.0.136
SINAC. (2016). Protocolo PRONAMEC: Protocolo para el Monitoreo Ecológico de las Formaciones Coralinas. Proyecto consolidación de las áreas marinas protegidas. Programa de Naciones Unidas para el Desarrollo (PNUD) y el Fondo para el Medioambiente Mundial (GEF). Costa Rica. Sin editorial.
SINAC-UNA. (2021). Protocolo Nacional para el Monitoreo Ecológico (PRONAMEC) de los Arrecifes Rocosos. Escuela de Ciencias Biológicas, UNA. Universidad Nacional. Costa Rica. Sin editorial.
Sokal, R. R. & Rohlf, F. J. (1981). Biometry: the principles and practice of statistics in biological research. EE. UU.: W.H. Freeman and Company.
Ulate, K., Alcoverro, T., Arthur, R., Aburto-Oropeza, O., Sánchez, C. & Huato-Soberanis, L. (2018). Conventional MPAs are not as effective as community co-managed areas in conserving top-down control in the Gulf of California. Biol. Conserv., 228, 100-109. https://doi.org/10.1016/j.biocon.2018.09.033
Ulate, K., Sánchez, C., Sánchez-Rodríguez, A., Alonso, D., Aburto-Oropeza, O. & Huato-Soberanis, L. (2016). Latitudinal regionalization of epibenthic macroinvertebrate communities on rocky reefs in the Gulf of California. Mar. Biol. Res., 12(4), 389-401. https://doi.org/10.1080/17451000.2016.1143105
van Oppen, M. J. H. & Gates, R. D. (2006). Conservation genetics and the resilience of reef building corals. Mol. Ecol., 15(13), 3863-3883. https://doi.org/10.1111/j.1365-294X.2006.03026.x
Wehrtmann, I. S. & Cortés, J. (Eds.) (2009). Marine Biodiversity of Costa Rica, Central America. Costa Rica: Springer.
Zgliczynski, B. J., Williams, G. J., Hamilton, S. L., Cordner, E. G., Fox, M. D., Eynaud, Y., … & Sandin, S. A. (2019). Foraging consistency of coral reef fishes across environmental gradients in the central Pacific. Oecologia, 191(2), 433-445. https://doi.org/10.1007/s00442-019-04496-9
ANNEX 1. List of macroinvertivores sampled in the Cabo Blanco Absolute Natural Reserve
ANEXO 1. Lista de macroinvertebrados muestreados en la Reserva Natural Absoluta Cabo Blanco
|
Trophic level |
Species |
Strata |
Trophic level |
Species |
Strata |
|||
|
Deep |
Shallow |
Deep |
Shallow |
|||||
|
Carnivores |
Aniculus elegans |
X |
Filter feeders |
Aplysina gerardogreeni |
X |
X |
||
|
Panulirus gracilis |
X |
Ascidian Morphotype sp1 |
X |
|||||
|
Stenorhynchus debilis |
X |
Bivalve Morphotype sp1 |
X |
|||||
|
Phataria unifascialis |
X |
Sponge Morphotype sp1 |
X |
|||||
|
Tambja abdere |
X |
Sponge Morphotype sp2 |
X |
X |
||||
|
Octopus sp |
X |
Sponge Morphotype sp3 |
X |
|||||
|
Hexaplex princeps |
X |
X |
Sponge Morphotype sp4 |
X |
X |
|||
|
Opeatostoma pseudodon |
X |
X |
Sponge Morphotype sp7 |
X |
X |
|||
|
Pustulatirus virginensis |
X |
X |
Sponge Morphotype sp8 |
X |
X |
|||
|
Triplofusus princeps |
X |
Sponge Morphotype sp11 |
X |
|||||
|
Turbo sp |
X |
Hyotissa hyotis |
X |
|||||
|
Herbivores |
Diadema mexicanum |
X |
X |
Ostrea sp. |
X |
X |
||
|
Eucidaris thouarsii |
X |
X |
Pinctada mazatlanica |
X |
X |
|||
|
Elysia diomedea |
X |
Pinna rugosa |
X |
|||||
|
Titanostrombus galeatus |
X |
Pycnoclavella stanleyi |
X |
|||||
|
Suspension feeders |
Carijoa riisei |
X |
Rhopalaea birkelandi |
X |
X |
|||
|
Leptogorgia alba |
X |
X |
Hete/Autotrophs |
Coral Morphotype Tubastrea |
X |
|||
|
Leptogorgia rigida |
X |
X |
Porites panamensis |
X |
||||
|
Aglaophenia whiteleggei |
X |
X |
N/D |
Gastropod Morphotype sp2 |
X |
X |
||
|
Tubastraea coccinea |
X |
Gastropod Morphotype sp3 |
X |
|||||
|
Neothyonidium sp |
X |
|||||||
|
Spirobranchius spp |
X |
X |
||||||
|
Terebellidae |
X |
X |
||||||
|
Vermetidae sp1 |
X |
|||||||
ANNEX 2. List of fish species sampled in the Cabo Blanco Absolute Natural Reserve
ANEXO 2. Lista de especies de peces muestreados en la Reserva Natural Absoluta Cabo Blanco
|
Trophic level |
Species |
Strata |
Trophic level |
Species |
Strata |
|||
|
Deep |
Shallow |
Deep |
Shallow |
|||||
|
Herbivores |
Acanthurus nigricans |
X |
X |
Macroinvertivores |
Alphestes inmaculatus |
X |
X |
|
|
Acanthurus xanthopterus |
X |
Anisotremus taeniatus |
X |
|||||
|
Kyphosus elegans |
X |
Arothron meleagris |
X |
X |
||||
|
Microspathodon dorsalis |
X |
X |
Bodianus diplotaenia |
X |
X |
|||
|
Ophioblennius steindachneri |
X |
X |
Canthidermis maculata |
X |
||||
|
Prionurus laticlavius |
X |
X |
Canthigaster punctatissima |
X |
||||
|
Prionurus punctatus |
X |
Chaetodon humeralis |
X |
X |
||||
|
Scarus ghobban |
X |
Cirrhitichthys oxycephalus |
X |
|||||
|
Scarus perrico |
X |
X |
Gnathanodon speciosus |
X |
||||
|
Scarus rubroviolaceos |
X |
Haemulon sexfasciatum |
X |
X |
||||
|
Piscivores |
Caranx caballus |
X |
Halichoeres chierchiae |
X |
||||
|
Caranx caninus |
X |
Halichoeres dispilus |
X |
|||||
|
Caranx melampygus |
X |
X |
Halichoeres nicholsi |
X |
||||
|
Caranx sexfaciatus |
X |
Holacanthus passer |
X |
X |
||||
|
Cephalopholis panamensis |
X |
Johnrandallia nigrirostris |
X |
|||||
|
Echidna nocturna |
X |
Paranthias colonus |
X |
|||||
|
Elagatis bipinnulata |
X |
Plagiotremus azaleus |
X |
|||||
|
Epinephelus labriformis |
X |
Pseudobalistes naufragium |
X |
|||||
|
Gymnothorax castaneus |
X |
X |
Stegastes flavilatus |
X |
X |
|||
|
Lutjanus argentiventris |
X |
Sufflamen verres |
X |
X |
||||
|
Lutjanus novemfasciatus |
X |
Thalassoma grammaticum |
X |
|||||
|
Plancktivores |
Abudefduf troschelii |
X |
X |
Thalassoma lucasanum |
X |
X |
||
|
Acanthemblemaria hancocki |
X |
X |
Trachinotus rhodopus |
X |
X |
|||
|
Chromis atrilobata |
X |
|||||||
|
Kyphosus ocyurus |
X |
|||||||
1 Sistema Nacional de Áreas de Conservación. andres.jimenez@sinac.go.cr ORCID: http://orcid.org/0009-0009-5291-7199 yamileth.cubero@sinac.go.cr ORCID: http://orcid.org/
2 Universidad Nacional, Costa Rica. fausto.arias.zumbado@una.cr ORCID: http://orcid.org/0000-0003-0391-592X andrea.garcia.rojas@una.ac.cr ORCID: http://orcid.org/ *
ORCID:




