
Revista UNICIENCIA
Uniciencia Vol. 38(1), January-December, 2024
E-ISSN: 2215-3470
DOI: https://dx.doi.org/10.15359/ru.38-1.30
Antimicrobial Activity of Diverse Chemotypes of Lippia graveolens Against Aeromonas hydrophila Isolated from Oreochromis niloticus
Actividad antimicrobiana de diversos quimiotipos de Lippia graveolens contra Aeromonas hydrophila aislada de Oreochromis niloticus
Atividade antimicrobiana de vários quimiotipos de Lippia graveolens contra Aeromonas hydrophila isolada de Oreochromis niloticus
Josué García-Pérez1, 2*, Juan Pérez-Sabino3, Susana Mendoza-Elvira4,
Antonio Jorge Ribeiro da Silva5, Juan Ulloa-Rojas6
Received: Aug/23/2023 • Accepted: Apr/16/2024 • Published: Aug/31/2024
|
Abstract [Objective] This study aimed to evaluate the antimicrobial efficacy of essential oil (EO) from diverse chemotypes of Lippia graveolens against oxytetracycline-resistant Aeromonas hydrophila, which primarily affects the tilapia aquaculture (O. niloticus) in Guatemala. [Methodology] L. graveolens were collected in three departments in Guatemala. The EO was obtained by hydrodistillation and characterized using gas chromatography and mass spectrometry (GC/MS). Subsequently, an antimicrobial assay was conducted using disk and dilution susceptibility tests and evaluation of synergistic interactions among the chemotypes. Each test was performed in triplicate. [Results] The analysis revealed the presence of twenty-seven compounds in the EO obtained from the chemotypes, with the main class being monoterpenes. The major constituents identified were cis-Dihydro-β-terpineol (8.84%) in chemotype I, carvacrol (51.82%) in chemotype II, and thymol (79.62%) in chemotype III. All EO chemotypes of L. graveolens demonstrated the ability to inhibit the A. hydrophila growth. Thymol chemotype exhibited the strongest inhibitory effect against bacterial growth, with a minimum inhibitory concentration (MIC) of 92.4 µg/mL and a minimum bactericidal concentration (MBC) of 184.8 µg/mL. Furthermore, the results suggest that there is no synergistic or additive effect when combining different chemotypes of L. graveolens. [Conclusions] This is the first report of L. graveolens chemotypes exhibiting antimicrobial activity against oxytetracycline-resistant A. hydrophila. The findings suggest that the chemotype thymol could be a potential treatment for infections in the tilapia aquaculture in Guatemala. Keywords: essential oil, oregano, tilapia, aquaculture, thymol Resumen [Objetivo] El objetivo de este estudio fue evaluar la eficiencia antimicrobiana del aceite esencial de diversos quimiotipos de Lippia graveolens contra la cepa Aeromonas hydrophila resistente a la oxitetraciclina, la cual afecta principalmente a la acuicultura de tilapia (O. niloticus) en Guatemala. [Metodología] L. graveolens se colectó en tres departamentos de Guatemala, los aceites esenciales (AE) se obtuvieron mediante hidrodestilación y se caracterizaron mediante cromatografía de gases y espectrometría de masas (GC/MS). Posteriormente, se realizó un ensayo antimicrobiano utilizando pruebas de susceptibilidad en disco y dilución, y se evaluaron las interacciones sinérgicas entre los diferentes quimiotipos. Cada prueba se repitió tres veces. [Resultados] El análisis evidenció la presencia de veintisiete compuestos en los AE obtenidos de los quimiotipos, siendo los monoterpenos la clase principal. Los principales constituyentes identificados fueron cis-dihidro-β-terpineol (8.84 %) en el quimiotipo I, carvacrol (51.82 %) en el quimiotipo II y timol (79.62 %) en el quimiotipo III. Todos los AE de los diferentes quimiotipos de L. graveolens demostraron capacidad para inhibir el crecimiento de A. hydrophila. En particular, el quimiotipo timol obtuvo el efecto inhibitorio más fuerte contra el crecimiento bacteriano, con una concentración mínima inhibitoria (CMI) de 92.4 µg/mL y una concentración mínima bactericida (CMB) de 184.8 µg/mL. Los resultados sugieren que no existe un efecto sinérgico o aditivo al combinar los diferentes quimiotipos de L. graveolens. [Conclusiones] Este estudio constituye el primer reporte sobre la actividad antimicrobiana de los diferentes quimiotipos de L. graveolens contra A. hydrophila resistente a la oxitetraciclina. Los hallazgos sugieren que el quimiotipo timol podría ser un tratamiento potencial para las infecciones en la acuicultura de tilapia en Guatemala. Keywords: Aceite esencial; orégano; tilapia; acuicultura; timol. Resumo [Objetivo] O objetivo deste estudo foi avaliar a eficiência antimicrobiana do óleo essencial de vários quimiotipos de Lippia graveolens contra a cepa Aeromonas hydrophila resistente à oxitetraciclina, que afeta principalmente a aquicultura de tilápia (O. niloticus) na Guatemala. [Metodologia] A L. graveolens foi coletada em três setores da Guatemala, os óleos essenciais (OE) foram obtidos por hidrodestilação e caracterizados por cromatografia a gás acoplada à espectrometria de massas (GC/MS). Posteriormente, foi realizado um ensaio antimicrobianos por meio de testes de suscetibilidade de disco e diluição, e foram avaliadas as interações sinérgicas entre os diferentes quimiotipos. Cada teste foi repetido três vezes. [Resultados] A análise revelou a presença de vinte e sete compostos nos OEs obtidos dos quimiotipos, sendo os monoterpenos a classe principal. Os principais constituintes identificados foram cis-di-hidro-β-terpineol (8,84%) no quimiotipo I, carvacrol (51,82%) no quimiotipo II e timol (79,62%) no quimiotipo III. Todos os OEs dos diferentes quimiotipos de L. graveolens mostraram a capacidade de inibir o crescimento de A. hydrophila. Em particular, o quimiotipo timol obteve o efeito inibitório mais forte contra o crescimento bacteriano, com uma concentração inibitória mínima (CIM) de 92,4 µg/mL e uma concentração bactericida mínima (CBM) de 184,8 µg/mL. Os resultados sugerem que não há efeito sinérgico ou aditivo ao combinar os diferentes quimiotipos de L. graveolens. [Conclusões] Este estudo é o primeiro relatório sobre a atividade antimicrobiana dos diferentes quimiotipos de L. graveolens contra A. hydrophila resistente à oxitetraciclina. Os resultados sugerem que o quimiotipo timol pode ser um tratamento em potencial para infecções na aquicultura de tilápia na Guatemala. Palavras-chave: Óleo essencial; orégano; tilápia; aquicultura; timol. |
In aquaculture practices in Guatemala, antibiotics have primarily been utilized for the therapeutic and prophylactic control of bacterial diseases. Many farmers believe that administering antibiotic therapy can provide significant advantages, such as reducing mortality rates resulting from bacterial infections. However, the repeated utilization of antibiotics has resulted in the emergence of drug-resistant bacteria, such as Aeromonas hydrophila (García-Pérez et al., 2021).
This bacterium can bioaccumulate in fishery products through the food chain and be consumed by the local community, causing bacteria resistance or harm to human health (Rigos et al., 2004; Romero et al., 2012; Alanazi et al., 2021). Therefore, to manage drug-resistant bacteria, it is necessary either to increase drug dosages or develop a new generation of antibiotics.
To prevent increasing drug dosages, livestock practices must change to treating diseases in fish, or a new alternative must emerge as an additional tool to control both resistant and non-resistant pathogens, as well as a means to reduce antibiotic usage. Medicinal plants can be one such alternative (Olusola et al., 2013; Ham et al., 2020).
The genus Lippia (Verbenaceae) is among the most used plants in Central and South America for this purpose (Pascual et al., 2001). This genus comprises around 200 species of herbs, shrubs, and small trees (Terblanché & Kornelius, 1996). In Guatemala, Lippia is represented by around 13 species, with L. graveolens, L. alba, L. salamensis, L. chiapasensis, L. dulcis, and L. cardiostegia being the most widely distributed (Standley & Williams, 1970).
Ethnobotanically, L. graveolens is commonly referred to as Mexican oregano. It is an aromatic perennial shrub that can grow up to 2 meters tall, with short-pilose branches and leaves on petioles typically measuring 5 mm–10 mm in length (Standley & Williams, 1970). The shrub is characterized by its axillary capitate spikes inflorescences, which consist of white, sessile, and zygomorphic flowers. These flowers are hermaphrodite and self-compatible (Ocampo-Velázquez et al., 2009).
Lippia graveolens is typically harvested from wild populations growing in a range of ecological conditions, from semi-arid to sub-humid lands. This can result in great morphological variability and chemical polymorphism, with essential oil composition being the most affected component (Tezara et al., 2014). Essential oils are found in all parts of most Lippia genus plants, but leaves or aerial parts are particularly rich in this component, as indicated by Pascual et al. (2001).
In Guatemala, researchers have identified different chemotypes of essential oils, namely carvacrol, thymol, and a minor type known as sesquiterpene (E)-caryophyllene. The latter is sometimes referred to as a mixed chemotype because it contains a variable combination of metabolites (Pérez-Sabino et al., 2012; Salgueiro et al., 2003). However, it is unknown whether the other chemotype with sesquiterpene as the major compound exists in Guatemala. To date, no research has been conducted on the antimicrobial properties of the EO chemotypes originated from wild populations of L. graveolens in Guatemala.
However, L. graveolens from various regions around the world has been investigated as a potential source of bioactive compounds possessing antibacterial and antioxidant properties, as well as for its potential use in preventing fish diseases (Bautista-Hernández et al., 2021; García-Pérez et al., 2019; Leyva-López et al., 2017). Several studies have evaluated the inhibitory effects of L. graveolens against a variety of bacteria, including Gram-positive strains such as Streptococcus agalactiae, Staphylococcus aureus, S. epidermidis, Sarcina lutea, Bacillus subtilis, and Enterococcus faecalis, as well as Gram-negative bacteria like Aeromonas hydrophila, A. sobria, Vibrio cholerae, Escherichia coli, Pantoea agglomerans, and Enterobacter aerogenes (Arana-Sánchez et al., 2010; Castellanos-Hernández et al., 2020; Hernández et al., 2009; Martínez et al., 2021).
Therefore, this study aimed to assess the antimicrobial properties of diverse chemotypes of L. graveolens grown wild in Guatemala, both individually and in combination, against the predominant bacterium in finfish aquaculture in Guatemala.
Samples of a wild population of Lippia graveolens (Verbenaceae) in Guatemala were collected from the following locations: I. El Subinal, Guastatoya, El Progreso (N14°51ˈ15 ̎ / W090°08ˈ05 ̎ [440 m.a.s.l]), II. El Carrizal, San Jacinto, Chiquimula (N14°37ˈ18 ̎ / W089°28ˈ58 ̎ [700 m.a.s.l]), and III. Casas de Pinto, Río Hondo, Zacapa (N15°01ˈ25 ̎ / W089°36ˈ35 ̎ [180 m.a.s.l]). Approximately 2–3 kg of raw materials (leaves) were collected between 7 a.m. and 10 a.m. at each site during the same week of sampling. The material was transported to the Aquatic Health Laboratory at San Carlos University for further analysis.
Its taxonomic identification was verified using Flora of Guatemala Part IX (Standley & Williams, 1970). Mr. Max Merida from San Carlos University authenticated the samples, and voucher specimens (USCG 48320, USCG 48321, and USCG 48322) were stored at the Herbarium USCG CECON-USAC.
The plant material was oven-dried at 45 °C for 48 hours, then ground into powder with an electric grinder. Then, the powder was sieved through a 300 µm–500 µm mesh and stored in a sealed bag at room temperature until further analysis.
Essential Oils Extraction and Gas Chromatography-Mass Spectrometry (GC/MS) Analysis to Determine the Chemotype of L. graveolens
A Clevenger-type device was used to extract essential oils (EO) by hydro-distillation (Majolo et al., 2018). For each extraction, 100 g of plant powder was added to a 2,000 mL volumetric flask and covered with tap water until submerged. The extraction process lasted 2 hours after the water began boiling, and the collected EO were stored at 4 °C in 10 mL amber glass bottles. This procedure was repeated three times.
The EO yield was calculated using the formula Y (%) = M/MV x 100, where M is the mass of EO obtained (g), and MV represents the amount of dried plant powder used (g). GC/MS analyses were performed using a Shimadzu 2010 Plus system coupled with a Shimadzu QP-2010 Plus selective detector (MSD), equipped with a DB5-MS capillary fused silica column (30 m, 0.25 mm I.D., 0.25 µm film thickness).
The oven temperature was set to increase from 60 ºC to 246 ºC at a rate of 3 ºC/min and then remained at 246 ºC for 20 minutes. He (99.999%) was used as carrier gas with a flow rate of 1.03 mL/min and a split ratio of 1:50.
Mass spectra were taken at 70 eV. The m/z values were recorded in the range of m/z 40–700 Da. EO components were identified by comparing their mass spectra and retention indices to values reported in the literature (Adams, 2007). Relative amounts of components were calculated based on GC peak areas without correction factors.
Bacterium Strain and Culture
Aeromonas hydrophila (strain: CEMA150219) was obtained from the bacterial collection of the Centro de Estudios del Mar y Acuicultura, Universidad de San Carlos de Guatemala. This bacterial strain was previously found in sick tilapia (O. niloticus) and has shown resistance to oxytetracycline, the most widely used antibiotic in tilapia farming in Guatemala.
To activate the strain, it was processed according to the National Committee for Clinical Laboratory Standards (NCCLS) standard method M31-T and considering the changes made by Alderman and Smith (2001).
The bacterium was cultured on TSA agar (TSA: Merck®) for 18 hours at 28°C. The temperature was selected according to Noga’s (2010) criteria, which recommend conducting microbiology studies of fish pathogens at the temperature at which the organisms are naturally found, rather than 37 °C, as the standard methodology indicates. This is because tropical or subtropical fish pathogens can exhibit poor growth or fail to grow under such conditions.
Subsequently, 3–5 colonies were identified and placed in 1X phosphate-buffered saline (PBS) until reaching a bacterial concentration of 1–2 x 108 CFU/mL, which was verified with an absorbance of 1.00 at a wavelength of 600 nm, measured using a spectrophotometer.
Disk Diffusion Method
Using the disk diffusion method according to Bauer et al. (1966) and considering the changes made by Mazumder et al. (2020), the study tested the effects of different EO chemotypes from L. graveolens on A. hydrophila. The chemotypes tested were: a) cis-Dihydro-β-terpineol [I], b) carvacrol [II], c) thymol [III]), as well as their combinations: d) [I:II], e) [I:III], f) [II:III] in a 50:50 ratio (v / v), and g) [I:II:III] in a ratio of 33:33:33 (v/v).
Within 15 minutes of preparation of the inoculum, a 100 µL aliquot of A. hydrophila strain was evenly spread on separate Muller-Hinton agar plates (MHA: Merck®). The aliquot was spread across the agar plate using an L-shaped loop, ensuring that it covered the entire diameter of the Petri dish.
Next, an empty paper disc (BBL: Sensi-Disc®) with a diameter of 6 mm was inoculated with 10 µL of each chemotype alone and in combination, and oxytetracycline discs (40 µg) were used as a positive control. Each EO and combination was tested in triplicate, with one disc placed in each Petri dish.
The plates with discs were incubated at 28 °C for 24 hours under aerobic conditions; after, the inhibition zone diameters (IZD) were measured with electronic calipers with an accuracy of 0.01 mm.
The interpretation of the antibacterial activity of EO was determined according to the criteria established by García-Pérez & Marroquín-Mora (2021), considering a specific plant extract, essential oil, or other natural product is considered to have antibacterial action when it produces IZD equal to or greater than 50% compared to the most used antibiotic. In Guatemala, oxytetracycline is the only antibiotic approved by the Guatemalan Ministry of Agriculture, Livestock and Food for tilapia aquaculture production.
Minimum Inhibitory (MIC) and Minimum Bactericidal Concentrations (MBC)
MIC and MBC determinations were conducted using the broth macrodilution assay according to NCCLS M31-T, with modifications by Alderman and Smith (2001). Two-fold serial dilutions of each essential oil chemotype of L. graveolens were prepared in the Muller-Hinton broth (MHB: Merck®) at concentrations ranging from 11.5 μg/mL to 11,825 μg/mL. Triton X (Sigma-Aldrich®) was used as the EO solubilizer at a concentration of 0.1% (v/v).
The A. hydrophila inoculum was adjusted to a concentration of 1 x 108 CFU/mL in PBS and then transferred into MHB to obtain a bacterial count of 1 x 105 CFU/mL. The tubes were then incubated at 28 °C for 24 hours under aerobic conditions. Finally, an aliquot of 100 µL from each tube was plated on TSA and incubated for 18 hours for bacterial count.
MIC and MBC were established according to Levison’s (2004) criteria. MIC was defined as the minimum concentration of antibiotic that prevents a clear suspension of 105 CFU/mL from becoming turbid after overnight incubation; turbidity generally connotes a 10-fold increase in bacterial density. MBC was determined to have the lowest concentration of antibacterial agent that reduces the viability of the initial bacterial inoculum to ≥ 99%.
Assessing Synergistic Interaction Among Chemotype of L. graveolens
The synergistic interaction among L. graveolens EO chemotypes was determined according to Nikkhah et al. (2017) and Gutierrez et al. (2008) with modifications. Four interactions were tested by combining three EO chemotypes: a) I:II, b) I:III, c) II:III, in a ratio of 50:50 (v/v) and combining the three EO chemotypes d) I:II:III in a ratio of 33:33:33 (v/v).
Once the independent MIC was determined, the fractional inhibitory concentration (FIC) was calculated as follows:
• FIC of chemotype cis-Dihydro-β-terpineol (FIC_I) = MIC (cis-Dihydro-β-terpineol[a, b]) in combination/MIC (cis-Dihydro-β-terpineol) alone.
• FIC of chemotype carvacrol (FIC_II) = MIC (carvacrol[a,c]) in combination/MIC (carvacrol) alone.
• FIC of chemotype thymol (FIC_III) = MIC (thymol[b,c]) in combination/MIC (thymol ) alone.
• FIC of all chemotypes (FIC_IV) = MIC (all chemotypes[d]) in combination/MIC (each chemotype) alone.
The FIC Index (FICi) was calculated as the sum of each FIC. The results were interpreted as follows: synergistic effect (FICi ≤ 0.5); additive effect (0.5< FICi ≤1); no interactive effect (1< FICi ≤4); antagonistic effect (FICIi > 4) as described by Gutierrez et al., (2008).
The EO yields and inhibition zone diameters for the disk diffusion assay were statistically analyzed with the nonparametric test of Kruskal Wallis, followed by the Mann-Whitney-Wilcoxon test (p < 0.05). The major compounds of the EO chemotypes from L. graveolens identified (greater than 1%) were used to determine the chemotaxonomy through Principal Component Analysis (PCA). Statistical analysis was conducted using statistical software (R Core Team, 2022).
The EO extracted from Lippia graveolens collected in Guatemala showed a yellow-reddish color, with yields significantly differing among departments (p < 0.05): 1.42 ± 0.25 % for El Progreso, 1.64 ± 0.14% for Chiquimula, and 3.77 ± 0.28 % for Zacapa. Therefore, it may be inferred that there is a relationship between EO origin and its total production.
These findings coincided with Martínez-Natarén et al. (2014), who reported that the EO yields of different plants varied according to the edaphoclimatic gradient. Therefore, different odors, colors, and amounts of metabolites may be found.
Concerning the chemotype aroma, a notable difference was observed. The EO chemotypes from Zacapa and Chiquimula had a spicy and intense pungent odor, while El Progreso chemotype had a less intense pungent odor.
Accordingly, based on odor, color, and consistency, it is inferred that the number of phenolic compounds may be higher in the Zacapa and Chiquimula chemotypes than in El Progreso. On the contrary, El Progreso may have a higher content of non-phenolic substances (oxygenated sesquiterpenes). The chromatographic profiles of each chemotype of L. graveolens are shown in Figure 1.
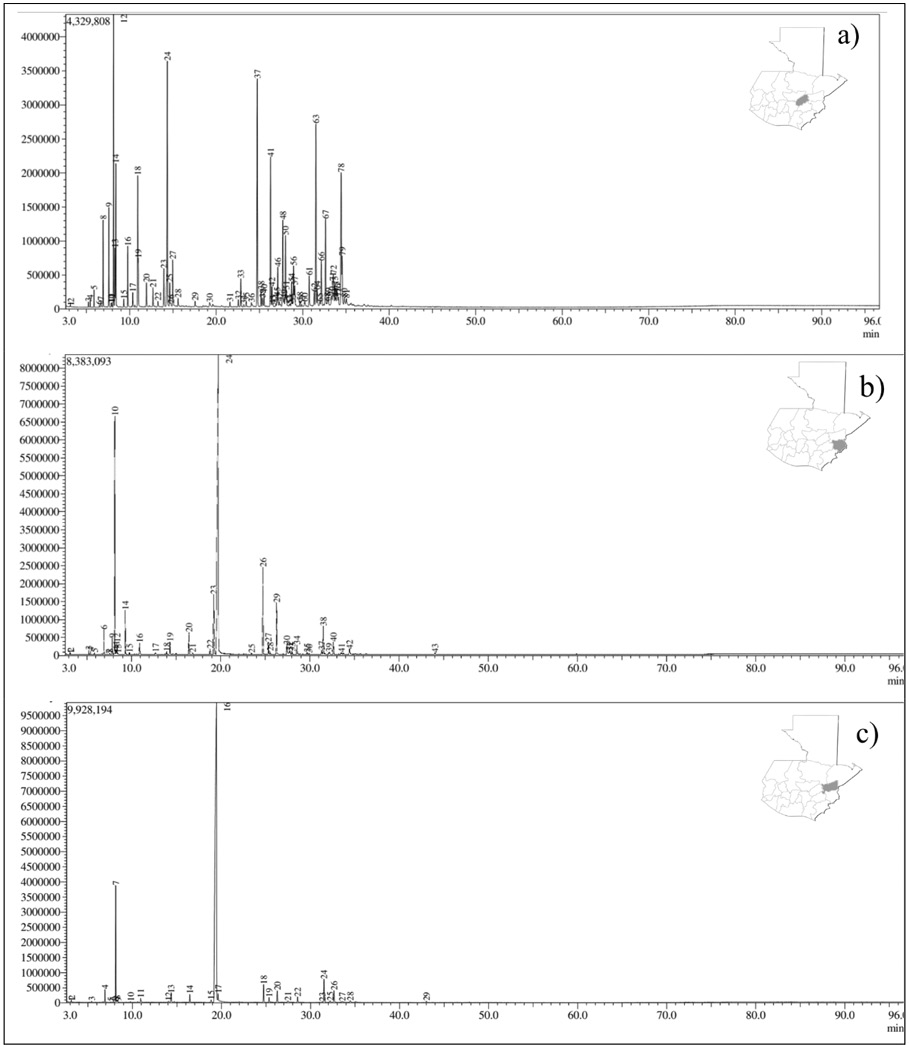
Figure 1. Derived from research. GC chromatogram of essential oils from Lippia graveolens collected from: a) El Progreso (I: cis-Dihydro-β-terpineol), b) Chiquimula (II: Carvacrol) and c) Zacapa (III: Thymol) departments.
According to Table 1, the content and proportion of chemical constituents of the EO (monoterpenes, sesquiterpenes, phenylpropanoids, and other compounds) varied among departments. The PCA horizontal axis explained 81.4% of the total variance, and the vertical axis explained 18.6% (Figure 2), suggesting the existence of three chemotypes (I: cis-Dihydro-β-terpineol: El Progreso, II. Carvacrol: Chiquimula, and III. Thymol: Zacapa) characterized by their different content of chemical metabolites.
Table 1. Chemical composition (%) of the three chemotype essential oils from Lippia graveolens
|
|
|
|
Chemotypes area (%) |
||||
|
No. |
Compounds* |
Type |
RT |
RI |
I |
II |
III |
|
1 |
Myrcene |
M |
6.918 - 6.926 |
988 |
1.82 |
1.13 |
- |
|
2 |
p-Cymene |
M |
8.121- 8.140 |
1022 |
7.22 |
13.94 |
7.76 |
|
3 |
Limonene |
M |
8.281 |
1024 |
1.40 |
- |
- |
|
4 |
1,8-Cineole |
M |
8.390 |
1026 |
3.54 |
- |
- |
|
5 |
γ-Terpinene |
M |
9.306 |
1054 |
2.28 |
2.38 |
- |
|
6 |
cis-Sabinene hydrate |
M |
9.776 |
1065 |
1.61 |
- |
- |
|
7 |
cis-β-Terpineol |
M |
10.925 |
1140 |
3.66 |
- |
- |
|
8 |
trans-Sabinene hydrate |
M |
10.984 |
1098 |
1.18 |
- |
- |
|
9 |
Isoborneol |
M |
13.944 |
1155 |
1.25 |
- |
- |
|
10 |
cis-Dihydro-β-terpineol |
M |
14.344 |
1156 |
8.84 |
- |
- |
|
11 |
δ-Terpineol |
M |
14.964 |
1162 |
1.66 |
- |
- |
|
12 |
Carvacrol, methyl ether |
M |
16.440 |
1241 |
- |
1.45 |
- |
|
13 |
Thymol |
M |
19.439 |
1289 |
- |
5.34 |
79.62 |
|
14 |
Carvacrol |
M |
19.750 |
1298 |
- |
51.82 |
- |
|
15 |
E-Caryophyllene |
M |
24.750 |
1417 |
8.74 |
6.53 |
1.65 |
|
16 |
α-Humulene |
S |
26.269- 26.280 |
1452 |
5.4 |
3.92 |
1.1 |
|
17 |
cis-Muurola-3,5-diene |
S |
27.117 |
1448 |
1.43 |
- |
- |
|
18 |
dehydro-Aromadendrane |
O |
27.700 |
1460 |
3.38 |
- |
- |
|
19 |
b-Chamigrene |
S |
27.996 |
1476 |
2.72 |
- |
- |
|
20 |
δ-Amorphene |
S |
28.936 |
1549 |
1.58 |
- |
- |
|
21 |
E-Nerolidol |
M |
30.754 |
1561 |
1.07 |
- |
- |
|
22 |
Caryophyllene oxide |
S |
31.515 - 31.526 |
1582 |
7.41 |
2.21 |
2.32 |
|
23 |
Hinesol |
S |
32.140 |
1640 |
1.95 |
- |
- |
|
24 |
Citronellyl pentanoate |
O |
32.636 - 32.640 |
1624 |
3.3 |
- |
1.2 |
|
25 |
γ-Eudesmol |
S |
33.544 |
1630 |
1.76 |
- |
- |
|
26 |
α-Eudesmol |
S |
34.444 |
1652 |
6.82 |
- |
- |
|
27 |
epi-α-Cadinol |
S |
34.556 |
1638 |
1.82 |
- |
- |
|
Total amount of monoterpene (%) |
44.27 |
82.59 |
89.03 |
||||
|
Total amount of sesquiterpene (%) |
30.89 |
6.13 |
3.42 |
||||
|
Total amount of other compounds (%) |
6.68 |
0 |
1.2 |
||||
|
Total amount of volatile compounds different chemotype of Lippia graveolens (%) |
81.84 |
88.72 |
93.65 |
||||
Note: derived from research. Type M: monoterpene, S: sesquiterpene, O: other compound, RT: retention time, RI: retention index, *Only compounds with a chromatographic area greater than 1% are listed in this table. Chemotypes I: cis-Dihydro-β-terpineol (El Progreso), II: carvacrol (Chiquimula), III: thymol (Zacapa) of Lippia graveolens.
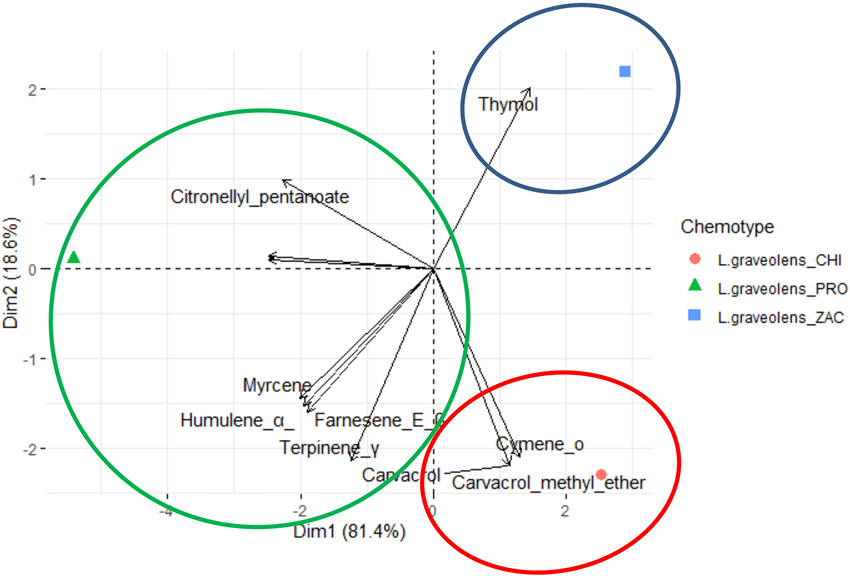
Figure 2. Derived from research. PCA of the 15 major components of the chemotypes among the departments PRO: El Progreso, CHI: Chiquimula and ZAC: Zacapa, of Lippia graveolens.
In general, twenty-seven compounds were identified in the EO of L. graveolens (Table 1), accounting for 81.84%, 88.72%, and 93.65% of the total EO composition of chemotypes I, II, and III, respectively. The monoterpenes constituted the main class, with cis-Dihydro-β-terpineol (8.84%) as a major constituent of chemotype I, carvacrol (51.82%) chemotype II, and thymol (79.62%) for chemotype III. Sesquiterpenes were the second major group of compounds found in the EO, with caryophyllene oxide as the major constituent of chemotypes I (7.41%) and III (2.32%), whereas the α-Humulene (3.92%) was the major constituent of chemotype II.
These results evidenced the EO chemical polymorphism of this species as mentioned previously for Guatemala (GT) and Mexico (MX): thymol (GT: 67.4%–73.5% / MX: 84.76%–91.77%), carvacrol (GT: 50.3%–51.8% / MX: 72.20%–83.49%), (E)-caryophyllene (GT:10.3%–12.3 % / MX: 0%), and caryophyllene oxide (GT: 2.7%–4.9% / MX: 4.94%–11.34%) (Martínez-Natarén et al., 2011; Pérez-Sabino et al., 2012; Salgueiro et al., 2003; Senatore & Rigano, 2001).
Pérez-Sabino et al. (2012) classified chemotype I as mixed because its heterogeneous compounds summed no more than 15% of the chemical structure. In this study, chemotype I had cis-Dihydro-β-terpineol (8.84%), E-Caryophyllene (8.74%), and p- Caryophyllene oxide (7.41%) as major constituents of the EO. These proportions differ from those reported previously from EO obtained in the same location. The differences in some compounds could be attributed to the genetic plasticity of L. graveolens, soil composition, climate variability, harvest time, and extraction method, among other factors (Benjemaa et al., 2022; Martínez-Natarén et al., 2014).
The results indicate that three EO chemotypes of L. graveolens provide promising alternatives for controlling the growth of Aeromonas hydrophila isolated from tilapia. Chemotypes III and II were the most effective, presenting IZD ranging from 28.46 mm to 34.03 mm (Table 2). Chemotype I exhibited the lowest IZD (9.95 mm). However, it is important to note that even though the IZD is the lowest, it still indicates an effect based on the criteria established in this study.
Table 2. Antibacterial activity of the three chemotype essential oils from L. graveolens against A. hydrophila
|
Chemotype combination (Ratio v/v) |
Inhibition zone diameter means ± standard deviation (mm) * |
MBC µg/mL |
MIC µg/mL |
FICi |
|
I |
09.95 ± 0.05 e |
2,956.3 |
1,478.1 |
- |
|
II |
28.46 ± 0.45 c |
369.5 |
184.8 |
- |
|
III |
34.03 ± 0.30 a |
184.8 |
92.4 |
- |
|
I : II (50:50) |
24.82 ± 0.40 d |
739.1 |
369.5 |
2.25 |
|
I : III (50:50) |
30.34 ± 0.48 b |
369.5 |
184.8 |
2.13 |
|
II : III (50:50) |
33.43 ± 0.58 a |
184.8 |
92.4 |
1.50 |
|
I : II : III (33:33:33) |
29.19 ± 0.20 c |
369.5 |
184.8 |
3.13 |
|
OXI |
10.32 ± 0.52 e |
For the combination of EO chemotypes, the highest IZD (29.19 mm–33.43 mm) was for II:III, followed by I:III and I:II:III. The lowest IZD (24.82 mm) was obtained for the combination of chemotype I:II. The positive control (OXI) had an IZD of 10.32 mm, indicating the resistance of the A. hydrophila to oxytetracycline.
The MIC and MBC values for the three EO chemotypes ranged from 92.4 µg/mL to 2,956.3 µg/mL (Table 2). The lowest MIC and MBC values were found for chemotype III (92.4 µg/mL–184.8 µg/mL), followed by chemotype II (184.8 µg/mL–369.5 µg/mL), and finally chemotype I (1,478.1 µg/mL–2,956.3 µg/mL). Although chemotype I obtained the highest MIC and MBC, it still may be considered effective. According to Bussmann et al. (2010), MIC values below 5,000 µg/mL are considered to exert strong antimicrobial activity.
Results indicated that the three EO chemotypes mixture from L. graveolens did not show a synergistic or additive effect. However, the greatest effect was observed when chemotype III was combined with any of the other chemotypes (I and II), even with chemotype I (with the lower inhibition capacity), thus exhibiting an antibacterial effect.
The EO extracted from chemotype III (thymol) exhibited better antibacterial properties than the EO components of chemotypes II and I, with significantly greater inhibition zone diameters and lower concentrations for MBC and MIC. Therefore, it may be inferred that the properties of the thymol metabolite could be responsible for the antibacterial activity observed in this study.
It is well documented that L. graveolens have an antibacterial effect against a broad group of pathogens, especially against Gram-negative bacteria (Arana-Sánchez et al., 2010; Bautista-Hernández et al., 2021; Hernández et al., 2009; Leyva-López et al., 2017; Salgueiro et al., 2003). However, this is the first report analyzing the effect of different EO chemotypes from L. graveolens growing in Guatemala against aquaculture oxytetracycline-resistant bacteria, such as A. hydrophila. It showed that secondary metabolites of L. graveolens may have a direct effect as antimicrobial agents, as stated by several authors for other plant species (Höferl et al., 2020; Koba et al., 2009; Nwanosike et al., 2016).
According to the results, most of the antimicrobial effects of the EO tested may be attributed to the presence and proportions of thymol and carvacrol in the three chemotypes, both acting independently and probably having the same action pathway, since there was no proven synergic or additive effect.
Although the antimicrobial effects of L. graveolens are well established, the mechanism of action is mostly unknown. Based on observations from the study by Lambert et al. (2001), it is inferred that the essential oil of L. graveolens can rupture the external membrane and may induce ATP permeability. This inference stems from the presence of thymol or carvacrol as the main components in L. graveolens. Furthermore, it was found that thymol and carvacrol could disintegrate the outer membrane of bacteria of A. hydrophila, isolated from different sources, and other types of bacteria, such as Escherichia coli and Salmonella typhimurium (Helander et al., 1998).
On the other hand, it is deduced that the components of chemotype I showed lower antibacterial activity due to their relative amount and type of sesquiterpenes, which have weak antimicrobial activity. This weak antimicrobial activity has been attributed to the fact that most sesquiterpenes do not have the appropriate hydrophobicity to break down the bacterial membrane (Bassole et al., 2005).
The results suggest that the essential oil from L. graveolens offers a promising alternative for controlling the growth of Aeromonas hydrophila. Three chemotypes were identified: I: cis-Dihydro-β-terpineol located in El Progreso Department, II: Carvacrol in the Chiquimula Department, and III: Thymol in the Zacapa Department. All EO exhibit antibacterial activities, albeit in different proportions. However, there was no synergistic or additive effect when combining different chemotypes.
The results suggest that the EO chemotype thymol can be used for treating bacterial infections in aquaculture. However, further studies are needed to assess the toxicity of L. graveolens EO on tilapia, as certain EO chemicals may directly impact the target bacteria, and larger amounts could potentially become toxic to fish and other organisms. Furthermore, it is recommended to investigate the in vivo efficacy of EO treatment for Aeromonas infections in finfish production in Guatemala.
This research constitutes a chapter of the first author’s doctoral thesis in the program ‘‘Doctorado en Ciencias Naturales para el Desarrollo (DOCINADE), Instituto Tecnológico de Costa Rica’’. It was funded by the Universidad de San Carlos de Guatemala cohort 2017. I want to express my deep appreciation to Carolina Marroquín for the accurate review of this document.
-The authors declare no competing interests.
All authors declare that they have read and approved the final version of this paper. The contribution percentages for this paper’s conceptualization, preparation, and correction are as follows: J.R.G.P 50%, J.F.P.S 10%, S.E.M.E 10%, A.R.S. 10%, and J.B.U.R. 20%.
The data supporting the results of this study will be made available by the corresponding author, J.R.G.P., upon reasonable request.
A preprint version of this article was deposited at: https://doi.org/10.1101/2023.07.24.549066
Adams, R. P. (2007). Identification of essential oil components by gas chromatography/mass spectroscopy. Allured Pub. Corp.
Alanazi, F., Almugbel, R., Maher, H. M., Alodaib, F. M., & Alzoman, N. Z. (2021). Determination of tetracycline, oxytetracycline, and chlortetracycline residues in seafood products of Saudi Arabia using high-performance liquid chromatography-photo diode array detection. Saudi Pharmaceutical Journal, 29(6), 566–575. https://doi.org/10.1016/j.jsps.2021.04.017
Alderman, D. J. & Smith, P. (2001). Development of draft protocols of standard reference methods for antimicrobial agent susceptibility testing of bacteria associated with fish diseases. Aquaculture, 196(1), 211–243. https://doi.org/10.1016/S0044-8486(01)00535-X
Arana-Sánchez, A., Estarrón-Espinosa, M., Obledo-Vázquez, E. N., Padilla-Camberos, E., Silva-Vázquez, R. and Lugo-Cervantes, E. (2010). Antimicrobial and antioxidant activities of Mexican oregano essential oils (Lippia graveolens H. B. K.) with different composition when microencapsulated in β-cyclodextrin. Letters in Applied Microbiology, 50(6), 585–590. https://doi.org/10.1111/j.1472-765X.2010.02837.x
Bassole, I. H. N., Nebie, R., Savadogo, A., Ouattara, C. T., Barro, N. and Traore, S. A. (2005). Composition and antimicrobial activities of the leaf and flower essential oils of Lippia chevalieri and Ocimum canum from Burkina Faso. African Journal of Biotechnology, 4(10), 1156–1160. https://www.ajol.info/index.php/ajb/article/view/71260
Bauer, A. W., Kriby, W. M. M., Sherris, J. C., Turck, M. (1966). Antibiotic Susceptibility Testing by a Standardized Single Disk Method. American Journal of Clinical Pathology, 45(4), 493–496, https://doi.org/10.1093/ajcp/45.4_ts.493
Bautista-Hernández, I., Aguilar, C. N., Martínez-Ávila, G. C. G., Torres-León, C., Ilina, A., Flores-Gallegos, A. C., Kumar Verma, D. and Chávez-González, M. L. (2021). Mexican oregano (Lippia graveolens Kunth) as source of bioactive compounds: A review. Molecules, 26(17), 1–19. https://doi.org/10.3390/molecules26175156
Benjemaa, M., Snoussi, M., Falleh, H., Hessini, K., Msaada, K., Flamini, G. and Ksouri, R. (2022). Chemical composition, antibacterial and antifungal activities of four essential oils collected in the North-East of Tunisia. Journal of Essential Oil Bearing Plants, 25(2), 338–355. https://doi.org/10.1080/0972060X.2022.2068971
Bussmann, R. W., Malca-García, G., Glenn, A., Sharon, D., Chait, G., Díaz, D., Pourmand, K., Jonat, B., Somogy, S., Guardado, G., Aguirre, C., Chan, R., Meyer, K., Kuhlman, A., Townesmith, A., Effio-Carbajal, J., Frías-Fernandez, F. and Benito, M. (2010). Minimum inhibitory concentrations of medicinal plants used in Northern Peru as antibacterial remedies. Journal of Ethnopharmacology 132(1), 101–108. https://doi.org/10.1016/j.jep.2010.07.048
Castellanos-Hernández, O. A., Rodríguez-Sahagún, Martha., Acevedo-Hernández, Gustavo., Aarland-Rayn, Clarenc. and Rodríguez-Sahagún, Araceli. (2020). Evaluation of the essential oil of Lippia graveolens as a growth inhibitor of Salmonella sp, E. coli and Enterococcus sp. E-CUCBA, 7(14), 1–6. https://doi.org/10.32870/e-cucba.v0i14.155
García-Pérez, J., Marroquín-Mora, D. and Pérez-González, M. (2019). Inclusión de extracto de Lippia graveolens (Kunth) en la alimentación de Oreochromis niloticus (Linnaeus, 1758) para la prevención de estreptococosis por Streptococcus agalactiae (Lehmann y Neumann, 1896). Revista AquaTIC, 54(1), 15–24. https://dialnet.unirioja.es/servlet/articulo?codigo=7681118#:~:text=La%20presente%20investigaci%C3%B3n%20evalu%C3%B3%20el%20efecto%20de%20la,L.%20graveolens%2C%20en%20una%20dieta%20comercial%20para%20tilapia.
García-Pérez, J. & Marroquín Mora, D. (2021). Evaluación in vitro de extractos de plantas medicinales como posibles agentes antimicrobianos para bacterias patógenas en tilapia. Kuxulkab', 27(57). 27-35. https://doi.org/10.19136/kuxulkab.a27n57.3702
García-Pérez, J. & Marroquín Mora, D. (2021). Evaluación in vitro de extractos de plantas medicinales como posibles agentes antimicrobianos para bacterias patógenas en tilapia. Kuxulkab', 27(57). 27-35. https://doi.org/10.19136/kuxulkab.a27n57.3702
García-Pérez, J., Ulloa-Rojas, J. B. and Mendoza-Elvira, S. (2021). Bacterial pathogens and their antimicrobial resistance in tilapia culture in Guatemala. Uniciencia, 35(2), 1–17. https://doi.org/10.15359/RU.35-2.4
Gutierrez, J., Barry-Ryan, C., and Bourke, P. (2008). The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. International Journal of Food Microbiology, 124(1), 91–97. https://doi.org/10.1016/j.ijfoodmicro.2008.02.028
Ham, Y., Yang, J., Choi, W. S., Ahn, B. J. and Park, M. J. (2020). Antibacterial activity of essential oils from Pinaceae leaves against fish pathogens. Journal of The Korean Wood Science and Technology, 48(4), 527–547. https://doi.org/10.5658/WOOD.2020.48.4.527
Helander, I. M., Alakomi, H.-L., Latva-Kala, K., Mattila-Sandholm, T., Pol, I., Smid, E. J., Gorris, L. G. M. and von Wright, A. (1998). Characterization of the action of selected essential oil components on Gram-negative Bacteria. Journal of Agricultural and Food Chemistry, 46(9), 3590–3595. https://doi.org/10.1021/jf980154m
Hernández, T., Canales, M., Avila, J. G., García, A. M., Meraz, S., Caballero, J. and Lira, R. (2009). Composition and antibacterial activity of essential oil of Lippia graveolens H.B.K. (Verbenaceae). Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas, 8(4), 295–300. https://www.redalyc.org/pdf/856/85611265010.pdf
Höferl, M., Wanner, J., Tabanca, N., Ali, A., Gochev, V., Schmidt, E., Kaul, V. K., Singh, V. and Jirovetz, L. (2020). Biological activity of Matricaria chamomilla essential oils of various chemotypes. Planta Medica International Open, 07(03), 114–121. https://doi.org/10.1055/a-1186-2400.
Koba, K., Poutoli, P. W., Raynaud, C., Chautmont, J. P. and Sanda, K. (2009). Chemical composition and antimicrobial properties of different basil essential oils chemotypes from Togo. Bangladesh Journal of Pharmacology, 4(1), 1–8. https://doi.org/10.3329/bjp.v4i1.998
Lambert, R. J. W., Skandamis, P. N., Coote, P. J. and Nychas, G.-J. E. (2001). A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. Journal of Applied Microbiology, 91(1), 453–462. https://doi.org/10.1046/j.1365-2672.2001.01428.x
Levison, M. E. (2004). Pharmacodynamics of antimicrobial drugs. Infectious Disease Clinics of North America, 18(3), 451–465. https://doi.org/10.1016/j.idc.2004.04.012
Leyva-López, N., Gutiérrez-Grijalva, E. P., Vazquez-Olivo, G. and Heredia, J. B. (2017). Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules, 22(6), 1–24. https://doi.org/10.3390/molecules22060989
Majolo, C., Pilarski, F., Chaves, F. C. M., Bizzo, H. R. and Chagas, E. C. (2018). Antimicrobial activity of some essential oils against Streptococcus agalactiae, an important pathogen for fish farming in Brazil. Journal of Essential Oil Research, 30(5), 388–397. https://doi.org/10.1080/10412905.2018.1487343
Martínez, J. Ramy., Chérigo, Lilia. & Ríos, Nivia. (2021). Evaluation of antibacterial properties of three Panamanian plants against multi-drug resistant bacteria. Tecnociencia, 23(1), 351–358. https://doi.org/10.48204/j.tecno.v23n1a19
Martínez-Natarén, D. A., Parra-Tabla, V., Dzib, G. and Calvo-Irabién, L. M. (2011). Morphology and density of glandular trichomes in populations of Mexican oregano (Lippia graveolens H.B.K., Verbenaceae), and the relationship between trichome density and climate. Journal of the Torrey Botanical Society, 138(2), 134–144. https://doi.org/10.3159/TORREY-D-10-00007.1
Martínez-Natarén, D. A., Parra-Tabla, V., Ferrer-Ortega, M. M. and Calvo-Irabién, L. M. (2014). Genetic diversity and genetic structure in wild populations of Mexican oregano (Lippia graveolens H.B.K.) and its relationship with the chemical composition of the essential oil. Plant Systematics and Evolution 300(3), 535–547. https://doi.org/10.1007/s00606-013-0902-y
Mazumder, A., Choudhury, H., Dey, A. and Sarma, D. (2020). Bactericidal activity of essential oil and its major compound from leaves of Eucalyptus maculata Hook. Against two fish pathogens. Journal of Essential Oil Bearing Plants. Plants, 23(1), 149–155. https://doi.org/10.1080/0972060X.2020.1729248
Nikkhah, M., Hashemi, M., Habibi Najafi, M. B. & Farhoosh, R. (2017). Synergistic effects of some essential oils against fungal spoilage on pear fruit. International Journal of Food Microbiology, 257(1), 285–294. https://doi.org/10.1016/j.ijfoodmicro.2017.06.021
Noga, E. (2010). Fish disease: Diagnosis and treatment. Iowa: Blackwell Publishing. https://doi.org/10.1002/9781118786758
Nwanosike, E., Fatokun, O. T., Okhale, S. E., Nwanosike, M. and Folashade Kunle, O. (2016). Phytochemistry and ethnopharmacology of Lippia genus with a statement on chemotaxonomy and essential oil chemotypes. International Journal of Pharmacognosy, 3(5), 201–211.
Ocampo-Velázquez, R. V., Malda-Barrera, G. X., Suárez-Ramos, G. (2009). Biología reproductiva del orégano mexicano (Lippia graveolens Kunth) en tres condiciones de aprovechamiento. Agrociencia, 43(5), 475–482.
https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-31952009000500003
Olusola, S. E., Emikpe, B. and Olaifa, F. (2013). The potentials of medicinal plants extracts as bio-antimicrobial in aquaculture. International Journal of Medicinal and Aromatic Plants, 3(3), 404–412.
Pascual, M. E., Slowing, K., Carretero, E., Sánchez Mata, D. and Villar, A. (2001). Lippia: traditional uses, chemistry and pharmacology: a review. Journal of Ethnopharmacology, 76(3), 201–214. https://doi.org/10.1016/S0378-8741(01)00234-3
Pérez-Sabino, J. F., Mérida-Reyes, M., Farfán-Barrera, C. D. and Ribeiro da Silva, A. J. (2012). Analysis and discrimination of the chemotypes of Lippia graveolens H.B.K. of Guatemala by solid phase microextraction, GC-MS and multivariate analysis. Química Nova, 35(1), 97–101. https://doi.org/10.1590/S0100-40422012000100018
R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Rigos, G., Nengas, I., Alexis, M., & Troisi, G. M. (2004). Potential drug (oxytetracycline and oxolinic acid) pollution from Mediterranean sparid fish farms. Aquatic Toxicology, 69(3), 281–288. https://doi.org/10.1016/j.aquatox.2004.05.009
Romero, J., Feijoó, C. G., & Navarrete, P. (2012). Antibiotics in Aquaculture-Use, Abuse and Alternatives. In E. Carvalho, G. David, & R. Silva (Eds.), Health and Environment in Aquaculture (IntechOpen).
Salgueiro, L. R., Cavaleiro, C., Gonc Ëalves, M. J., Proenc, A. and da Cunha, A. (2003). Antimicrobial activity and chemical composition of the essential oil of Lippia graveolens from Guatemala. Planta Medica, 69(1), 80–83. https://doi.org/10.1055/s-2003-37032
Senatore, F. and Rigano, D. (2001). Essential oil of two Lippia spp. (Verbenaceae) growing wild in Guatemala. Flavour and Fragrance Journal. J, 16(3), 169–171. https://doi.org/10.1002/ffj.972
Standley, Paul., & Williams, Louis. (1970). Flora of Guatemala Part IX. In Standley, P. C., Williams, L. O. and Gibson, D. N. (Eds.), Flora of Guatemala (I, Vol. 24). Field Museum of Natural History. https://doi.org/10.5962/bhl.title.2434
Terblanché, F. C. and Kornelius, G. (1996). Essential Oil Constituents of the Genus Lippia (Verbenaceae) A Literature Review. Journal of Essential Oil Research, 8(5), 471–485. https://doi.org/10.1080/10412905.1996.9700673
Tezara, W., Coronel, I., Hererra, A., Dzib, G., Canul-Puc, K., Calvo-Irabién, L. M. and González-Meler, M. (2014). Photosynthetic capacity and terpene production in populations of Lippia graveolens (Mexican oregano) growing wild and in a common garden in the Yucatán peninsula. Industrial Crops and Products, 57, 1–9. https://doi.org/10.1016/j.indcrop.2014.03.012
1* Corresponding author
Josué García-Pérez,
 josuegarciap@profesor.usac.edu.gt,
josuegarciap@profesor.usac.edu.gt,  https://orcid.org/0000-0002-6899-8036
https://orcid.org/0000-0002-6899-8036Juan Pérez-Sabino,
 fpsabino@yahoo.com,
fpsabino@yahoo.com,  https://orcid.org/0000-0001-5736-0371
https://orcid.org/0000-0001-5736-0371Susana Mendoza-Elvira,
 seme@unam.mx,
seme@unam.mx,  https://orcid.org/0000-0003-3672-6471
https://orcid.org/0000-0003-3672-6471Antonio Jorge Ribeiro da Silva,
 ajorge@ippn.ufrj.br,
ajorge@ippn.ufrj.br,  https://orcid.org/0000-0002-7579-420X
https://orcid.org/0000-0002-7579-420XJuan Ulloa-Rojas,
 juan.ulloa.rojas@una.cr,
juan.ulloa.rojas@una.cr,  https://orcid.org/0000-0003-4464-1136
https://orcid.org/0000-0003-4464-1136Doctorado en Ciencias Naturales para el Desarrollo (DOCINADE), Instituto Tecnológico de Costa Rica, Universidad Nacional, Universidad Estatal a Distancia, Costa Rica.
2 Centro de Estudios del Mar y Acuicultura, Universidad de San Carlos de Guatemala, Guatemala, Guatemala.
3 Escuela de Química, Facultad de Ciencias Químicas y Farmacia, Universidad de San Carlos de Guatemala, Guatemala, Guatemala.
4 Facultad de Estudios Superiores Cuautitlán, Universidad Nacional Autónoma de México, Estado de México, México.
5 Instituto de Pesquisas de Produtos Naturais, Universidade Federal do Rio de Janeiro (UFRJ), Brazil.
6 Escuela de Ciencias Biológicas, Universidad Nacional, Heredia, Costa Rica.
Antimicrobial Activity of Diverse Chemotypes of Lippia graveolens Against Aeromonas hydrophila Isolated from Oreochromis niloticus (Josué García-Pérez • Juan Pérez-Sabino • Susana Mendoza-Elvira • Antonio Jorge Ribeiro da Silva • Juan Ulloa-Rojas) Uniciencia is protected by Attribution-NonCommercial-NoDerivs 3.0 Unported (CC BY-NC-ND 3.0)
URL: www.revistas.una.ac.cr/uniciencia
Correo electrónico: revistauniciencia@una.cr


