
Rev. Ciencias Veterinarias, Vol. 39, N° 1, [1-14], E-ISSN: 2215-4507, enero-junio, 2021
DOI: https://doi.org/10.15359/rcv.39-1.2
URL: http://www.revistas.una.ac.cr/index.php/veterinaria/index
Anatomy of the Respiratory System and Heart of the Sloth (Choloepus hoffmanni) of Costa Rica
Anatomía del sistema respiratorio y del corazón del perezoso (Choloepus hoffmanni) de Costa Rica
Anatomia do sistema respiratorio e do coração do bicho-preguiça (Choloepus hoffmanni) de Costa Rica
Daira Jimena Vigil-Esquivel1 , Sofía Coto-Sanabria1, Jazmín Fiorella Vega-Alfaro1, Estefanía Cendra-Villalobos1, Rebeca Melissa Viloria-Hernández1, Laura Chaverri-Esquivel1, Andréia Passos-Pequeno1
, Sofía Coto-Sanabria1, Jazmín Fiorella Vega-Alfaro1, Estefanía Cendra-Villalobos1, Rebeca Melissa Viloria-Hernández1, Laura Chaverri-Esquivel1, Andréia Passos-Pequeno1
 Autor de correspondencia: daira.vigilesquivel@hotmail.com
Autor de correspondencia: daira.vigilesquivel@hotmail.com
1 Department of Anatomy, School of Veterinary Medicine, Universidad Nacional, Costa Rica.
Emails: daira.vigilesquivel@hotmail.com; soficotosanabria10@gmail.com; jaz.vega.2711@gmail.com; estef.villa.una@gmail.com; mevih96@gmail.com; laura.chaverri.esquivel@una.cr; andreia.passos.pequeno@una.cr
Received: June 24, 2020 Corrected: March 1, 2021 Accepted: March 12, 2021
Abstract
The two-toed sloth (Choloepus hoffmanni) faces various diseases related to the respiratory and circulatory system. Thus, prior knowledge of anatomy becomes relevant since it contributes to the study and interpretation of medical images and surgical procedures. This study aims to describe the topographic and macroscopic anatomy of the heart and the respiratory system. Three animals were preserved with a supersaturated saline solution. Throughout the dissection, it was possible to identify and describe the components of the respiratory system and the heart. Some of the particularities found were the division of the frontal sinus in several sections. In addition, the presence of a large thyroid cartilage and the absence of wedge-shaped processes in the epiglottis were observed. The heart was small, although with very prominent auricles. These findings should be considered in the performance of clinical-surgical procedures and in the interpretation of medical images.
Keywords: anatomy, Choloepus hoffmanni, heart, respiratory system
Resumen
El perezoso de dos dedos (Choloepus hoffmanni) se enfrenta a diversas enfermedades relacionadas con el sistema respiratorio y circulatorio. Por lo tanto, el conocimiento previo de la anatomía se vuelve relevante ya que contribuye al estudio e interpretación de imágenes médicas y procedimientos quirúrgicos. Este estudio tiene como objetivo describir la anatomía topográfica y macroscópica del corazón y del sistema respiratorio. Tres animales fueron preservados con una solución salina sobresaturada. A lo largo de la disección, fue posible identificar y describir los componentes del sistema respiratorio y el corazón. Algunas de las particularidades encontradas fueron la división del seno frontal en varias secciones. Además, se observó la presencia de un cartílago tiroideo grande y la ausencia de procesos en forma de cuña en la epiglotis. El corazón era pequeño, aunque con aurículas muy prominentes. Estos hallazgos deben considerarse en la realización de procedimientos clínico-quirúrgicos, así como en la interpretación de imágenes médicas.
Palabras claves: anatomía, Choloepus hoffmanni, corazón, sistema respiratorio
Resumo
O bicho-preguiça de dois dedos (Choloepus hoffmanni) é afetado por várias doenças relacionadas ao sistema respiratório e circulatório. Portanto, o conhecimento prévio da anatomia torna-se relevante, pois contribui para o estudo e interpretação de imagens médicas e procedimentos cirúrgicos. Este estudo tem como objetivo descrever a anatomia topográfica e macroscópica do coração e do sistema respiratório. Três animais foram preservados com solução salina supersaturada. Ao longo da dissecção, foi possível identificar e descrever os componentes do sistema respiratório e do coração. Algumas das peculiaridades encontradas foi a divisão do seio frontal em várias seções. Além disso, foi observada a presença de uma cartilagem tireóide grande e a ausência de processos em forma de cunha na epiglote. O coração era pequeno, embora com aurículas muito proeminentes. Esses achados devem ser considerados na realização de procedimentos clínico-cirúrgicos, bem como na interpretação de imagens médicas.
Palavras-chave: anatomia, Choloepus hoffmanni, coração, sistema respiratório
Introduction
The C. hoffmanni belongs to the superorder Xenarthra, which also includes anteaters and armadillos, order Pilosa, and belongs to the family Megalonychidae. The sloths are widely distributed, considering that they can be found in South and Central America (Gilmore et al., 2001; Torres-Trujillo & Mantilla-Meluk 2016). They are usually perceived as unique and captivating animals; therefore, they face risky situations such as hunting and illegal traffic. Nevertheless, they are not included in the International Union for Conservation of Nature (IUCN) list as an endangered species. This contributes to the increase of their presence in wildlife centers, places where is required information available to give them the necessary care regardless of being medical, nutritional, etc. Studies about the anatomy of the respiratory system and the heart will allow us to provide relevant information for better knowledge about this species; this knowledge can help with the improvement of the conservation, management, and clinical care programs.
The sloths are susceptible to various diseases that might affect the respiratory and circulatory systems. Some of them are very frequent in this species, such as pneumonia. Thus, the knowledge of anatomy could be of great help for interpreting imaging tests and serving as a basis for a necropsy.
A study of the anatomy of the respiratory system and heart was conducted to describe the gross anatomy and topographical relationship between its components; thus, it provided information to assist in the medical and diagnostic assistance to these Xenarthras
Materials and methods
Three specimens of C. hoffmanni were used in this study; all of them were females of unknown age. They were donated by the Hospital for Small and Wild Species of the National University of Costa Rica during the second semester of 2019. The cause of death for two of the sloths was trauma; for the third specimen, there is no data of the cause of death.
Before starting with the dissection, all specimens were weighed, measured, and shaved with a shaving machine, so as not to contaminate the interior with hairs and better visualize the anatomy of the specimens. The preservation of the cadavers was done by using a supersaturated saline solution, injected into the common carotid artery, and keeping them under refrigeration during the study (Figure 1).

The specimens were placed in dorsal decubitus for the dissection of the thoracic cavity, starting with an incision from the intermandibular joint until the pubis, following the ventral midline. The skin and muscles were reflected, thusly the organs were exposed, studied in situ and, then, removed for further examination ex situ. During the study, a photographic record (Huawei Mate 10 Lite) was made.
Results
Respiratory System. The structures of the respiratory system were identified by making a section across the skull. The frontal sinus, the sphenoid sinus, the nasal septum, the nasal turbinates (dorsal, middle, and ventral), and the nasal meatuses (dorsal, middle, and ventral) were visible, along with the hard and soft palates, oropharynx, and nasopharynx (Figure 2).

Anatomy of the upper respiratory system. The upper part of the respiratory system comprises the nose, nasal cavity, turbinate and meatuses, and the paranasal sinuses. The nose is the external part. It is characterized by being flattened, naked, and has two rigid-edged nostrils attached to the upper lip (Figure 3), which communicates with the nasal cavity. This cavity is limited dorsally by the nasal bone, ventrally by the palatine process of the incisive bone and the palatine bone and, finally, is limited laterally by maxillary bones. Also, it is completely divided into the left and right part of the nasal septum that runs from the ethmoid bone to the dorsal part of the hard palate. Likewise, the nasal cavity is divided into the vestibule, which is rostral, and the nasal cavities, as the caudal part.

The lumen of the nasal cavity contains three nasal turbinates: dorsal, middle, and ventral (Figure 2). They are ossified and located dorsal to the hard palate. The dorsal and ventral nasal turbinate are situated more rostrally compared to the middle one; however, the middle turbinate is characterized by being larger. In each of the cavities, there are spaces in the middle of the nasal turbinates; these correspond to the meatus. Therefore, there is the presence of a dorsal nasal meatus, a ventral nasal meatus, a medial nasal meatus, and a common nasal meatus (Figure 2).
Besides, the paranasal sinuses are air-filled spaces in the skull, and it was possible to identify some of them. The frontal sinuses, located in the frontal bone, are characterized by the presence of several septa that separate them into cavities. Furthermore, the sphenoid sinus was identified, lying into the sphenoid bone as a single cavity (Figure 2).
At last, still on the head, a hard palate was observed marked by the presence of transverse ridges and continuing with a soft palate. This soft palate performs the division of the pharynx into the oropharynx and nasopharynx.
Anatomy of the lower respiratory system. The lower part of the respiratory system comprises the larynx, trachea, and lungs.
The larynx is composed of four cartilages (Figure 4). The epiglottis is a single cartilage that has a rounded leaf shape and is the most rostral of the cartilages. It is located at the beginning of the larynx vestibule and is characterized by the absence of the cuneiform processes. Between the epiglottis and the arytenoid cartilage are the aryepiglottic folds, which extend from the lateral sides of the epiglottis to the arytenoid cartilage. The arytenoid cartilage is a pair structure and is characterized by the presence of corniculate processes.

The thyroid cartilage is very large and is the ventral and lateral part of the larynx. It is formed by two lateral plates united in the ventral part, and it is the lateral limit for the piriform recess. Likewise, the cricoid cartilage is located caudally in the larynx; the median crest and its ring shape could be observed (Figure 4).
As part of the respiratory system, the larynx is connected to the trachea (Figure 4), located caudally to the cricoid cartilage. The trachea consists of a series of tracheal rings that are open on the dorsal area; in this area is positioned the tracheal muscle. The trachea passes through the visceral space ventral to the cervical spine accompanied by the esophagus; the cervical part of the trachea keeps in the median plane, ventral to this it is located the sternohyoid muscle. However, the thoracic part rotates to the right slightly until the bifurcation in the fourth or fifth intercostal space. The cranial vena cava accompanies this part of the trachea.
The bifurcation leads to the origin of two main bronchi, one left and one right, which communicate with the lungs. Inside the lungs, they branch out into a bronchial tree. This constitutes the basis for naming the lung lobes. However, in this study, it was not possible to perform techniques for filling the bronchial tree followed by corrosion; therefore, external segments of each lung were identified according to the presence of pulmonary fissures.
The pleura covers the lobes; the color of the parenchyma is between red and marron and has a smooth appearance. The lobes of each lung are arranged in a certain way. The right lung is lobed into the cranial, caudal, and accessory lobes. In contrast, the left lung is divided into the caudal lobe, the cardiac notch, and the cranial lobe that is divided into two portions: the caudal and cranial. The pulmonary hilum is the site through which structures, such as veins, arteries, nerves, and lymphatic vessels, pass.
Heart and great vessels. Regarding the heart, it is covered by the pericardium and is located in the thoracic cavity (Figure 5). With the heart still in situ, joined to the lungs by the pulmonary arteries (left and right), the main structures were identified, such as its atriums and ventricles. The heart, alongside the great vessels, was disinserted to visualize the arteries, left subclavian, pulmonary trunk, and arteries that irrigate certain thoracic areas such as the internal thoracic, vertebral, and deep cervical artery.

After identifying the previously mentioned structures, the heart of C. hoffmanni was located in the thoracic cavity, in the medial mediastinum region, and was perceived to have the shape of an inverted raindrop. Additionally, it was determined that the heart was slightly diverted to the left side of the body’s midline and had a slanted direction, meaning the right atrium leaned towards the right side cranially, and the apex of the heart leaned towards the left side caudally. The base of the heart was directed cranially towards the cranial lobe of the right lung, while the caudal edge leaned towards the caudal lobe of the right lung (Figure 6).
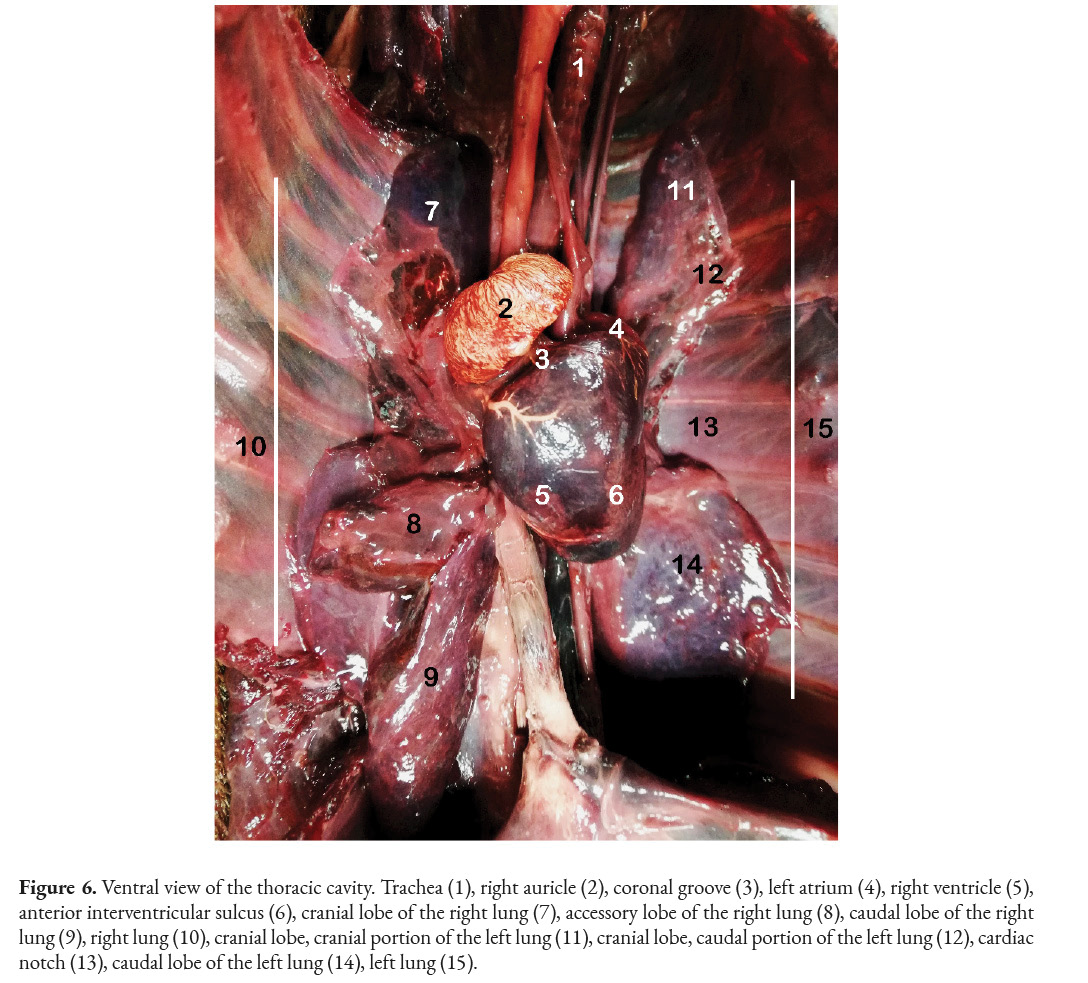
The heart was located behind approximately four intercostal spaces; also, the fibrous pericardium that covers the heart joins to the diaphragm through the pericardiophrenic ligament. Additionally, the heart was separated from the lungs. The internal comparison of the heart of C. hoffmanni was made by an incision in the left ventricle; using this method made it possible to identify the chordae tendineae and the internal appearance of the left auricle with its pectineus muscles that project towards the inside. On the other hand, the venae by the visceral pleura.
Furthermore, after just cutting open the thoracic cavity, the heart had a viscose appearance and a dark red color. Likewise, the heart has two halves, the arterial and the venous, with their respective left and right cardiac chambers; both auricles of C. hoffmanni were prominent. The left and right halves were divided by the interventricular septum. Also, from the right surface, the subsinuosal interventricular groove could be observed, while from the left side, the anterior interventricular groove could be visualized.
The internal composition of the heart of C. hoffmanni was made by an incision in the left ventricle. Using this method made it possible to identify the chordae tendineae and the internal appearance of the left auricle with its pectineus muscles that project towards the inside and, the venae cavae more the coronary sinus lead to the right atrium.
The left atrium’s wall has a smooth appearance, excepting shallow diverticula located in the mid-wall, formed by muscle bands. These diverticula can also be found in the truncus pulmonalis and the left pulmonary artery (Rowlatt 1980).
The left atrioventricular opening has an oval shape and is protected by a valve composed of the septal and parietal cusps with their respective semilunar valvula, according to Rowlatt (1980).
The left ventricle has the following general characteristics, according to Rowlatt (1980): its cavity has the shape of a wide cone, and most of its wall has trabeculae, except for the subaortic region. It is also possible to observe that the anterior papillary muscles can be double, while the posterior papillary muscles join the ventricle’s wall. In addition, the pars membranacea is located between the septal cusp and the aortic valve on its posterior region; these are not formed by cartilage or bone.
The aortic orifice is guarded by the aortic valve, which is tricuspid; this means it has three semilunar valvulae, the septal, left, and right cusps. The ostium of the left and right coronary arteries may arise deep into the aortic sinuses.
Moreover, from the aortic arch emerge the left subclavian artery and the brachiocephalic trunk, the latter continues as the bicarotid trunk, which divides into the common carotid arteries, left and right. The brachiocephalic trunk is also the origin of the right subclavian artery (López & Mayor s.f.).
Discussion
During the dissection of the three C. hoffmanni specimens, it was observed that the limits of the nasal cavity were consistent with what was described in domestic animals. Furthermore, in domestic animals, the paranasal sinuses are invaginations in the skull bones from the nasal cavity; also, in domestic animals, the frontal, maxillary, palatine, sphenoid, and lacrimal sinuses are identified (in ruminants and pigs) (König & Liebich 2020). However, by making a section across the skull of one of the C. hoffmanni specimens, only two paranasal sinuses were identified: the frontal sinus between the nasal and cranial cavity, and the sphenoid sinus, below where the optic chiasm would be located.
Likewise, Dünner & Pastor (2017) described that this species is macrosmatic, meaning it has a highly sensitive sense of smell; this agrees with the results of this research evidenced in its nose, which is highly developed and prominent, unlike Bradypus variegatus that has a slightly prominent nose with 3-4 mm diameter nostrils. Furthermore, the presence of sweat in the nose of C. hoffmanni demonstrates the presence of sweat glands in this region, resembling bovines that have many serous glands (Dünner & Pastor 2017; König & Liebich 2020) (Figure 3).
There are similarities between the nasal turbinates found in the C. hoffmanni and the ones described in domestic animals (König & Liebich 2020), since this species has three nasal turbinates, and there is great development of the middle nasal turbinate resembling carnivores like cats and dogs. These authors also mention that pigs and cattle have a partitioned frontal sinus, which is also true in this species of two-toed sloth (Figure 2).
It is of great importance to know the anatomy of the paranasal sinuses because during an infectious illness, such as sinusitis caused by microorganisms like Enterobacter cloacae, for example, abundant thick mucus can accumulate in the sinuses. It can also be a sign of more serious problems at the level of lower airways, such as the lungs, where the pathogen may have colonized and caused pneumonia since all these structures and organs are anatomically interconnected (Dünner & Pastor 2017).
The topography of the larynx and trachea in C. hoffmanni agrees with what was described for this species (Dünner & Pastor 2017), which does not differ from other mammals. Therefore, the location of these structures is the same as in domestic animals (König & Liebich 2020). In addition, the presence of the sternohyoid muscle in C. hoffmanni resembles that of domestic mammals. However, the trachea of this species differs from that of B. variegatus since the latter has a long trachea that reaches the diaphragm, giving a 360º turn in the distal portion and rotating cranially again to branch into the primary bronchi so that it can rotate the neck in different directions and extend the trachea without collapsing (Britton 1941; Dünner & Pastor 2017).
Likewise, the larynx of C. hoffmanni agrees with that of domestic animals regarding its tubular and hollow shape, in addition to the cartilage that gives it its shape (König & Liebich 2020), with the only difference that no vocal cords were found as well as in B. variegatus. Despite the absence of vocal cords, the sloths can emit sounds such as guttural grunts and “hissing” sounds by diverting air loudly through the nostrils; these sounds are important in the communication between the juvenile and its mother and the agonistic behaviors between adults defending the territory or their young (Blanco & Jones 2014; Dünner & Pastor 2017; Pessoa et al., 2018). The trachea of C. hoffmanni has the same components as that of domestic mammals; however, its length differs from what was indicated for this species (Dünner & Pastor 2017), it is shorter, and the carina is not at the level of the fifth intercostal space but at the entrance to the thoracic cavity. Also, the C. hoffmanni trachea only divides into both primary bronchi without a tracheal bronchus observed in cattle and pigs (König & Liebich 2020).
The topography of the lungs in the dissected sloths was the same as the described for domestic animals (König & Liebich 2020). Some authors point out that the adult C. hoffmanni does not have lobed lungs, but a groove that divides them (Dünner & Pastor 2017), similar to the one in the lungs of B. variegatus, which only divide into cranial and caudal lobes (Britton, 1941). However, some authors affirm that C. hoffmanni has lobed lungs (López & Mayor s.f.), and this is what was found in the dissected C. hoffmanni specimens. The cause of this disagreement is assumed to be the age of the animal; it was young since it had a thymus. Thus, only young individuals have lobed lungs; a groove only separates them as they grow. Also, the lobulation of the left lung is the same as in carnivores and pigs (König & Liebich 2020), while the composition of the right lung disagrees with that of domestic mammals due to the absence of the middle lobe (Figure 7).

It should be noted that this pulmonary lobulation found in the dissected specimens is described as seen in the external appearance of the lungs and their fissures, since there were no available methods for airway repletion. However, the external appearance of lungs is also very important to locate anatomical structures in radiographic studies and for their auscultation (Hammond et al., 2009) and identify the site of affection of pathology. At the same time, aspects such as the color of the lung parenchyma must be uniform and between reddish and maroon as it is described in dissected specimens; this allows identifying pathologies, such as paracoccidioidomycosis caused by the fungus Paracoccidioides brasiliensis, which causes white granuloma-like lesions of firm consistency, approximately 1-3 cm in diameter in the lung parenchyma (Trejo-Chávez et al., 2011). It is relevant to mention that the noted color of the lung parenchyma in the dissected specimens could be darker than the present in alive animals due to post-mortem changes since the specimens were found and until the conservation technique was applied to them (Ibargoyen, 2015).
Sloths are heterothermal animals (Munaó & Oliveira 1999); this means they are really sensitive to temperature changes, which is also why they don’t inhabit high areas. According to Dünner & Pastor (2017), the body temperature of Choloepus ranges between 34°C and 37°C; however, according to Pauli et al. (2016), who made an estimate, the minimum should be 33°C and the maximum 37°C, with an overall temperature range of 2.5° C (Pauli et al., 2016; Dünner & Pastor, 2017). Colder temperatures make these animals more susceptible to acquiring a pathogen (Hammond et al., 2009). For treatment, it is essential to maintain the sloth’s temperature and humidity at the correct parameters, which are 25-27°C and 80-90% of humidity (Dünner & Pastor 2017). Due to their heterothermic nature, they have a slow metabolism and can survive long periods of apnea, which can last around 10 – 40 minutes, and which occurs by modifying the blood supply to tissues, allowing greater blood supply to the brain (Irving et al., 1942; Munaó & Oliveira, 1999; Dünner & Pastor, 2017). During a study of 81 B. variegatus and C. hoffmanni sloths, it was noted that most of them acquired pneumonia during winter and springtime (Munaó & Oliveira, 1999). The sloths were lethargic, malnourished and had an occasional nasal discharge. The diagnosis was based on lung auscultation and clinical signs. A sloth with pneumonia caused by the bacteria Enterobacter cloacae and Corynebacterium sp. has various abscesses in its lungs (Dünner & Pastor 2017). This kind of respiratory disease can be easily prevented if the sloths in captivity are kept with the correct standards such as temperatures and nutrition and always close attention to their exhibit’s ambiance.
The irrigation of the lungs in C. hoffmanni and B. variegatus does not differ from other species despite the prominent size of the atriums of their heart (Dünner & Pastor 2017). For this reason, the pulmonary circulation in the study of this species is very similar to that described for domestic animals (König & Liebich 2020).
In this study, the anatomical location of the heart of C. hoffmanni, the number of chambers, and its pericardial lining are the same as what was described for domestic animals (König & Liebich 2020); however, unlike these ones, this organ is not between the 3rd and 6th ribs (7th in the case of carnivores), but more caudal. It does not have as much fat in the epicardium. It differs in size compared to the body weight of the animal (Dünner & Pastor 2017); similarly, in B. variegatus, it represents only 0.2% of their body weight.
The heart’s small size compared to C. hoffmanni’s body weight may be the reason why these animals have a slow lifestyle and cannot maintain rapid movements for long periods (Dünner & Pastor 2017). This anatomical characteristic leads to the heart rate being in a lower range that goes from 40 to 90 beats per minute (Dünner & Pastor 2017), while in domestic animals, it goes from 60 to 180 beats per minute in dogs and from 140 to 220 beats per minute in cats (Torrente & Bosch 2012). These are important considerations for C. hoffmanni clinical analysis since these are parameters that must be controlled during anesthesia because it tends to decrease up to 4 beats per minute (Lescano et al., 2014). They are also widely used parameters to identify clinical signs of infectious processes caused by pathogens such as B. bronchiseptica that causes mild bradycardia (Hammond et al., 2009).
Besides, according to what Dünner & Pastor (2017) described for this species, this study also revealed that the C. hoffmanni has internal characteristics considered as primitive, such as the size of its auricles and the shape of the atrioventricular valves. The external shape of the heart, broad base and apex formed by the left ventricle (Hayssen 2011), was confirmed; however, according to what was observed, it differs from some authors who wrote about having a double caudal vena cava (Dünner & Pastor 2017). Irrigation of the heart is consistent with that of domestic mammals (König & Liebich 2020).
In addition, a distinctive characteristic of the heart and its vessels are the branches that emerge from the aortic arch in the species B. variegatus (Albuquerque et al., 2018), opposed to those that originate in C. hoffmanni’s aortic arch. The aortic arch in B. variegatus is the origin of the left subclavian artery, the left common carotid artery, and the brachiocephalic trunk, which divides into the right common carotid artery and the right subclavian artery. On the other hand, the bicarotid trunk in C. hoffmanni will start the left and right common carotid arteries. There is no evidence that the presence of the bicarotid trunk in the two-toed sloth is linked with a predisposition to cardiac pathologies. Cases of sloths with cardiac diseases have been found; however, all of them seem to be isolated occurrences (Chetboul et al., 2017). Even though other sloth species like B. variegatus don’t have a bicarotid trunk, certain domestic species such as ruminants, pigs, equines, and alpacas do, which makes this finding not that uncommon (Pérez et al., 2017; König & Liebich, 2020).
Conclusion
The results obtained offer a deepening of the knowledge of the morphology of the respiratory system and the heart of C. hoffmanni. As a result, it was possible to demonstrate the direct relationship between the anatomy and physiology of this species, specifically in the organs studied, which are perfectly adapted to the behavior and metabolism of this species. In addition, this study offers an important subsidy for performing clinical-surgical procedures and interpreting medical images.
Acknowledgments
Conflict of interest statement
None of the authors has any conflicts of interest to declare.
References
Albuquerque, P.V., Sena, D.G.F., Braz, R.S., Mesquit, E.P., Lacerda, M.A.S., Silva, W.J., Sá, F.B. & Amorim, M.J.A.A.L. (2018). Ramos do arco aórtico e da aorta torácica em bicho-preguiça (Bradypus variegatus). Arq. Bras. Med. Vet. Zootec. 70(4), 1203–1211. https://doi.org/10.1590/1678-4162-9963
Blanco, R.E. & Jones, W.W. (2014). Estimation of hearing capabilities of Early Miocene sloths (Mammalia, Xenarthra, Folivora) and palaeobiological implications. Hist Biol 28(3), 390-397. https://doi.org/10.1080/08912963.2014.946415
Britton, S. W. (1941). Form and function in the sloth. Q Rev Biol 16(1), 13-34.
Chetboul, V., Gouni, V., Tissier, R., Jiménez, M., Huynh, M., Pouchelon, J.L. & Chai, N. (2017). Feasibility, Within-Day and Between-Day Variability of Transthoracic Echocardiography in Sloths (Bradypus variegatus and Choloepus hoffmanni). J Vet Sci Med Diagn. 6(5), 4. https://doi.org/10.4172/2325-9590.1000245
Dünner, C. & Pastor, G. (2017). Manual de manejo, medicina y rehabilitación de perezosos. Fundación Huálamo, Chile. p. 154.
Gilmore, D.P., Da Costa, C.P. & Duarte, D.P. (2001). Sloth biology: an update on their physiological ecology, behavior, and role as vectors of arthropods and arboviruses. Braz J. Med Biol Res 34(1), 9-25. https://doi.org/10.1590/s0100-879x2001000100002
Hammond, E. E., Sosa, D., Beckerman, R. & Aguilar, R. F. (2009). Respiratory Disease Associated with Bordetella bronchiseptica in a Hoffmann’s Two-Toed Sloth (Choloepus hoffmanni). J Zoo Wildl Med 40(2), 369-372. https://doi.org/10.1638/2008-0086.1
Hayssen, V. (2011). Choloepus hoffmanni (Pilosa: Megalonychidae). J Mammal 43(873): 37-55. https://doi.org/10.1644/873.1
Ibargoyen, G.S. (2015). Cambios Post Mortem Generales. http://www.produccion-animal.com.ar/veterinaria_forense/15-cambiospm.pdf (Accessed February 23, 2021).
Irving, L., Scholander, P. & Grinnel, S. (1942). Experimental studies of the respiration of sloths. J Cell Comp Physiol 20(2), 183-210. https://doi.org/10.1002/jcp.1030200207
König, H.E. & Liebich, H.G. (2020). Veterinary Anatomy of Domestic Mammals: Textbook and Colour Atlas (7th Edition). Thieme, New York. p. 397-418, 471-500.
Lescano, J., Quevedo, M., Baselly, C. & Fernández, V. (2014). Inmovilización química reversible de corta duración en perezosos de dos dedos (Choloepus didactylus) cautivos empleando ketamina, xilacina y midazolam. Rev Investig Vet Peru 25(2), 171-181.
López, C. & Mayor, P. (s.f.). Atlas de anatomía de especies silvestres de la Amazonia peruana. https://atlasanatomiaamazonia.uab.cat/atlas.asp?texto=3 (Accessed June 10, 2020).
Munaó, L. & Oliveira, P. (1999). Clinical problems of sloths (Bradypus sp. and Choloepus sp.) in captivity. J Zoo Wildl Med 30(1), 76-80.
Pauli, J. N., Peery, M. Z., Fountain, E. D. & Karasov, W. H. (2016). Arboreal Folivores Limit Their Energetic Output, All the Way to Slothfulness. Am Nat 188(2), 196–204. https://doi.org/10.1086/687032
Pérez, W., Méndez, V., Vazquez, N., Navarrete, M. & König, H. E. (2017). Gross anatomy of the heart of the alpaca (Vicugna pacos, Linnaeus 1758). Anat Histol Embryol 47(2), 110–118. https://doi.org/10.1111/ahe.12327
Pessoa, E., Zevêdo, A. & Soares, C. (2018). Agonistic interactions in the brown-throated three-toed sloth, Bradypus variegatus (Pilosa: Bradypodidae), in an urban environment in Rio Tinto, Paraíba, Brazil. Edentata 19, 42–46. https://doi.org/10.2305/IUCN.CH.2018.EDENTATA-19-1.5.en
Rowlatt, U. (1980). Functional and Nonfunctional Determinants of Mammalian Cardiac Anatomy, Parts I and II. In: Bourne, G. (Ed.). Comparative Anatomy and Development. Academic Press, New York, p. 259-300. https://doi.org/10.1016/B978-0-12-119401-7.50014-1
Torrente, C. & Bosch, L. (ed.). (2012). Medicina de urgencia en pequeños animales (Tomo I). Servet, Zaragoza. p. 360.
Torres-Trujillo, N. & Mantilla-Meluk, H. (2016). Anotaciones sobre la distribución del perezoso de dos dedos Choloepus hoffmanni (Pilosa: Megalonychidae) para el departamento del Quindío, en las listas de mamíferos de Colombia. Investigación, Biodiversidad y Desarrollo 35(1), 20-30.
Trejo-Chávez, A., Ramírez-Romero, R., Ancer-Rodríguez, J., Nevárez-Garza, A.M. & Rodríguez-Tovar, L.E. (2011). Disseminated Paracoccidioidomycosis in a Southern Two-Toed Sloth (Choloepus didactylus). J Comp Pathol 144(2-3), 231-234. https://doi.org/10.1016/j.jcpa.2010.08.012
 Licencia Creative Commons Atribución-No-Comercial SinDerivadas 3.0 Costa Rica
Licencia Creative Commons Atribución-No-Comercial SinDerivadas 3.0 Costa Rica