
Rev. Ciencias Veterinarias, Vol. 40, N° 2, [1-16], E-ISSN: 2215-4507, julio-diciembre, 2022
DOI: https://doi.org/10.15359/rcv.40-2.4
URL: http://www.revistas.una.ac.cr/index.php/veterinaria/index
Human ascariasis, an evolutionary complex relationship between host and parasite
Ascariasis humana, una relación evolutivamente compleja entre el huésped y el parásito
Ascaridíase humana, uma relação evolutivamente complexa entre hospedeiro e parasito
Mario Baldi1 , Norberto Baldi2
, Norberto Baldi2
1 Programa de Investigación en Enfermedades Tropicales, Escuela de Medicina Veterinaria, Universidad Nacional, Heredia, Costa Rica; mario.baldi.salas@una.ac.cr  0000-0001-6109-4993
0000-0001-6109-4993
2 Laboratorio de Antropología Biológica. Escuela de Antropología. Universidad de Costa Rica, San Pedro, Costa Rica; norberto.baldi@ucr.ac.cr  0000-0003-3279-2949
0000-0003-3279-2949
 Autor de correspondencia: mario.baldi.salas@una.ac.cr
Autor de correspondencia: mario.baldi.salas@una.ac.cr
Recibido: 10 de setiembre de 2021 Corregido: 25 de mayo de 2022 Aceptado: 31 de mayo de 2022
Abstract
Ascariasis is a debilitating parasitic disease that has decimated the health of hundreds of thousands of human beings, especially in developing countries. The lack of adequate prophylaxis of the parasite associated with an increased risk of immunological disease is a challenge mainly in developed countries where the prevalence of this parasite is very low. The evolutionary relationship and mechanisms by which the parasite was able to colonize and establish itself in human hosts have not yet been unraveled. In addition, the host immune response mechanisms to eliminate or control the parasite are not fully understood. Understanding these immunological mechanisms (proximates) will allow establishing better medical treatments for diseases related to the positive effects of parasitosis, thus also avoiding the undesirable effects of the parasitosis itself.
Keywords: Evolution, Ascariasis, Zoonosis, Ascarid therapy, Ascaris suum, Ascaris lumbricoides.
Resumen
La ascariasis es una enfermedad parasitaria que diezma la salud de cientos de miles de seres humanos, especialmente en los países en desarrollo. Sin embargo, la falta de profilaxis adecuada del parásito, asociado con un mayor riesgo de sufrir enfermedades inmunes, es un problema principalmente en los países desarrollados donde la prevalencia del parásito es muy baja. La relación evolutiva y los mecanismos por los cuales el parásito fue capaz de colonizar y establecerse en el huésped humano aún no se han descifrado. Del mismo modo, los mecanismos inmunes (proximales) de respuesta del huésped para eliminar o controlar el parásito no se entienden completamente. La comprensión de estos mecanismos nos permitirá establecer mejores tratamientos médicos, evitando efectos indeseables y más enfocados en el control de la parasitosis, así como para la terapia de estas enfermedades inmunológicas asociadas con la relación huésped-ascárido.
Palabras clave: Evolución, Ascariasis, Zoonosis, Terapia de Ascarídios, Ascaris suum, Ascaris lumbricoides
Resumo
A ascaridíase é uma doença parasitária que dizima a saúde de milhares de seres humanos, principalmente nos países em desenvolvimento. No entanto, a falta de profilaxia adequada do parasito, associada ao aumento do risco de doenças imunológicas, é um problema principalmente em países desenvolvidos onde a prevalência do parasito é muito baixa. A relação evolutiva e os mecanismos pelos quais o parasito foi capaz de colonizar e se estabelecer no hospedeiro humano ainda não tem sido decifrados. Da mesma forma, os mecanismos de resposta imune do hospedeiro para eliminar ou controlar o parasito não são totalmente compreendidos. A compreensão desses mecanismos permitirá estabelecer melhores tratamentos médicos, evitando efeitos indesejáveis e mais voltados para o controle das parasitoses, bem como para a terapia dessas doenças imunológicas associadas à relação hospedeiro-ascarídeo.
Palavras-chave: Evolução, Ascaridíase, Zoonoses, Terapia Ascaridea, Ascaris suum, Ascaris lumbricoides
Introduction
Ascariasis is a disease in humans caused by ubiquitous gastrointestinal parasites known as Ascaris lumbricoides and occasionally also by Ascaris suum (Leles et al., 2012; Loreille and Bouchet, 2003). Ascariasis is one of the most widespread diseases worldwide, especially in tropical and subtropical geographical areas. It is estimated that between 1.2 and 1.5 billion people are affected, with 100-200 million individuals (mostly children) showing clinical symptoms. Poverty, lack of drinking water, poor sanitary and hygienic conditions are among the factors associated with its high transmission in regions with high prevalence (Betson et al., 2014; Dold and Holland, 2011; Loreille and Bouchet, 2003; Peng y Criscione, 2012). Ascariasis is considered a neglected tropical disease, among other parasitic diseases, prevalent mostly in developing countries (Dold and Holland, 2011).
Ascaris lumbricoides Linnaeus, 1758 and Ascaris suum Goeze, 1782 are morphologically indistinguishable (Betson et al., 2014) and have a genetic difference of only six nucleotides in their ITS-1 segment. Although classified by some authors as two distinct species, they are taxonomically very close to each other (Leles et al., 2012; Peng and Criscione, 2012). For example, both species have a monoxen-direct transmission life cycle (Criscione et al., 2007; Dold and Holland, 2011; Roberts et al., op. 2009) and identical disease mechanisms.
Economic losses associated with ascariasis can be quantified by counting disability-adjusted life years (DALYs = 10.5 million) (Barendregt, 2002; Dall’ Orso et al., 2014). If we add the high morbidity rate associated with clinical diseases (~122 million cases/year), this disease has profound effects on both the public health system and the local economy (O’Lorcain and Holland, 2000) given treatment costs and the loss of working hours. On the other hand, some evidence suggests that this parasitosis may have beneficial effects, especially in reducing the incidence or development of immune-mediated diseases (Briggs et al., 2016; Correale, 2014). It has been hypothesized that this apparent positive effect in infections is related to a host-parasite evolution (Parker and Ollerton, 2013). This situation leaves a gap regarding the most appropriate treatment, the development of new therapies, and the control over this parasitosis in humans and animals based on evolutionary medicine.
When Homo sapiens met Ascaris lumbricoides:
Several hypotheses attempt to explain the evolutionary relationship between A. lumbricoides and Homo sapiens (Criscione et al., 2007; Leles et al., 2012; Loreille and Bouchet, 2003). A very close evolutionary link has been suggested between A. lumbricoides and A. suum given their genetic similarity, life cycle resemblance, and cross-infections to humans and pigs (Loreille and Bouchet, 2003; Nejsum et al., 2012). Fossil records of ascarids in humans date back to around 30,000 years ago. However, since these records are inconsistent, it cannot be ensured that they are unique to H. sapiens given the possibility of contamination with eggs of ascarids of other mammals (e.g. Ursus speleacus) (Leles et al., 2012). Certainly, pig domestication, linked to the emergence of ascariasis in humans, has not yet been fully elucidated. Palaeontological evidence suggests that pigs diversified into different groups: in the Americas in the late Eocene (34 million years ago) and in Europe, Asia, and Africa in the Oligocene (23 million years ago). On the other hand, large hominids emerged between 24 and 16 million years ago, followed by hominins who diverged from a common ancestor between 9 and 7 million years ago (Dunsworth, 2010). This suggests that humans and wild pigs coexisted in the same environment for a long time, being an important food source for early hunter-gatherers in Eurasia (Leles et al., 2012), thus opening up the possibility of ascariasis spillover through close contact between these two species.
Four hypotheses have been suggested for the origin of A. lumbricoides (Figure 1). The first hypothesis proposes that both Ascaris spp shared a common ancestor, possibly before the domestication of pigs (S. scrofa) around 10,000-9,000 years ago in the Near East (Larson et al., 2005; Rito et al., 2019). However, the lack of evidence of ascariasis in great apes and the low diversity of Ascaris in Old World primates place this hypothesis in a weak position (Leles et al., 2012).
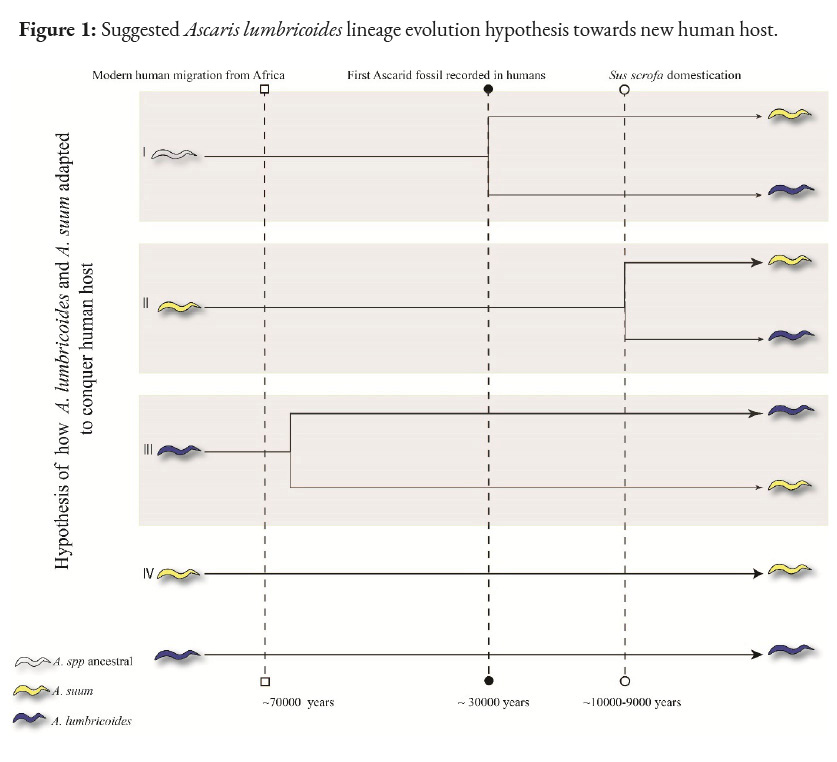
The second hypothesis states that A. lumbricoides derives directly from A. suum through an allopatric speciation event favoring host shift (pig to human) and that the current pig Ascaris is its persistent ancestor from the former (Liu et al., 2012). This origin has been suggested in the Neolithic, when pig domestication took place and, consequently, human populations increased, allowing the maintenance of the endemic cycle through epidemics (threshold host density) (Holt et al., 2003). Furthermore, at least two divergence events have been proposed for such parasite colonization, the first occurring ~15,000-2,500 years ago based on mitochondrial phylogeny (origin of haplotype cluster C, exclusive in European pigs) and a second more recent event, ~1,800-300 years ago (origin of haplotype clusters B and A, predominant in pigs in China and Africa) (Betson et al., 2014; Nejsum et al., 2017). Multiple successful host colorizations in different areas associated with human migrations suggested that such human populations might have been the starting point for the establishment of the definitive host shift (Zhou et al., 2011). This idea is based on the comparison of the evolutionary process that Trichocephalus trichiurus in humans originated from Trichuris suis (pig definitive host). However, this parallelism loses strength when comparing the criteria used for Trichocephalus trichiurus, as these hardly fit the criteria for the origin of A. lumbricoides. For example, Ascaris spp. are not very diverse in Old World primates, contrary to Trichuris spp. The higher rate survival of A. lumbricoides in humans (long-lived) suggests a better ecological adaptation to the human host compared to A. suum, which contrasts with reports on T. suis and T. trichiurus. Finally, the absence of robust paleontological material confirming the existence of A. lumbricoides in human settlements prior to pig domestication differs from the evidence for T. trichiura (Leles et al., 2012; Loreille and Bouchet, 2003). The lack of consensus on the natural ability of A. lumbricoides to effectively infect pigs (only with high parasite loads of A. lumbricoides) and the inability of A. suum to reach a mature stage in the human host are the most relevant arguments weakening this hypothesis (Leles et al., 2012).
Another possibility is that A. suum derived directly from A. lumbricoides, the former being the most recent species. Under this hypothesis, an allopatric event occurred, but from humans to pigs, which is debated based on the long association between early hominins in Africa and subsequent early human migrations. The spread of these ascarids to new geographical areas simultaneously occurring with pig domestication would provide the opportunity to jump to the new host species, but the absence of fossil records to support this idea weakens this third hypothesis (Betson et al., 2014; Leles et al., 2012; Loreille and Bouchet, 2003).
The hypothesis that both species (L. lumbricoides and summs) are conspecific is based on the low genetic divergence in several genetic markers (ITS1 of the rDNA) between the two species (Cavallero et al., 2013; Criscione et al., 2007; Leles et al., 2012). Experimentally and naturally, cross-infection between A. suum and A. lumbricoides has been achieved in the opposite hosts, thus completing the cycle in both cases. However, some authors have not been able to complete the A. lumbricoides cycle in pigs, arguing that very high parasite loads are required to achieve this infection in this species (Easton et al., 2020). This contradiction in this type of experimental infection has not allowed for a consensus on the true transmission capacity between these species. In addition, this hypothesis contradicts those who defend that both species are really one.
The argument to classify them as a single species is based on the low genetic composition plus their little morphological differentiation between these two nematodes, especially when they share the same geographical location. But others argued that this short difference in genetic makeup is enough to classify these species as divergent species (Leles et al. 2012). Betson et al. molecularly density analyzed ascarids samples from humans and pigs from around the world (mDNA, microsatellite markers), concluding that there are less than 10% marked differences between these two species worldwide, which was not sufficient for other authors (Betson et al., 2014; Dold and Holland, 2011; Easton et al., 2020; Leles et al., 2012) to classify each of them into two distinct species. More recently DNA sequencing of the complete mitochondrial genome confirmed the existence of the previously described haplotype clusters (A, B, and C) using the mitochondrial marker cox-1 (Betson et al., 2014; Cavallero et al., 2013; Nejsum et al., 2012; Nejsum et al., 2017).
Genetic evidence of speciation:
It is more complicated to clarify whether these two species are assigned the status of a single species or conversely two different species using molecular markers to achieve the identification of the genotype. There are two haplotypes, one related to humans and the other to Ascaris in pigs. Anderson et al. showed that the mtDNA allozyme allele divergence in both clades is around 3-4%, showing that each of them has independent vital cycles, and, as a result, they are two different species (Anderson et al., 1993) a difference that was eventually corroborated by other authors using more accurate molecular markers (Betson et al., 2014; Peng and Criscione, 2012; Zhou et al., 2011).
Cavallero et al. used restriction fragment length polymorphism to identify the nuclear ITS (ITS1, 5.8S, ITS2) region as a target for the pig and human Ascaris-specific marker. These analyses identified five parasitic genotypes. Genotype 1 was associated with humans, genotype 2 with pigs, while the other genotypes were found in both species. The sharing of genotypes between these two species would be explained by an evolutionary process such as introgression or retention of ancestral polymorphisms (Cavallero et al., 2013; Liu et al., 2012; Nadler and Hudspeth, 2000; Peng et al., 2003; Zhou et al., 2011). This means that, when hybridization between close relatives or when sister taxa diverge, they need an extended period of time to be truly genetically distinguished from each other (Anderson, 2001).
However, this marker (ITS-1) has a high intra-individual variability, which hinders its use as a reliable diagnostic marker when applied to close relatives species (Anderson, 2001; Leles et al., 2012). Given that the complete sequencing of the mitochondrial genome (mtDNA) of A. lumbricoides shows a 1.9% difference with the A. suum genome, it is considered a single species (Leles et al., 2012; Liu et al., 2012). Finally, the phylogenetic relationship of the ascarids established by Nadler and Hudspeth (Nadler y Hudspeth, 2000) using a parsimony analysis between morphology and genetic markers concludes that both species are sister taxa given the short pairwise distance, and that the most likely recent common ancestor is Parascaris equorum (equine ascarid) (Leles et al., 2012; Nadler and Hudspeth, 2000). Interbreeding between A. suum and A. lumbricoides has been observed in both China (7.8%) and Guatemala (4%) from the 23 microsatellite markers, but not in other sympatric geographic areas for these two sympatric species. Although the existence of inbreeding is confirmed, the fitness of these hybrids appears to be very low; otherwise, the population structure in the hybrid would have been established in these populations(Betson et al., 2014; Holland and Smith, 2005; Zhou et al., 2011). However, the inconsistency to establish a clear separation or non-separation between these two species is related to the selection of molecular diagnostic markers, by not including several sequences of loci, which can be achieved by means of next generation sequence methods and increasing the number of samples from different geographic areas in the world (Anderson, 2001; Easton et al., 2020). In this way, the difference between these species could be established in an adequate and definitive manner (Holland and Smith, 2005; Leles et al., 2012; Peng and Criscione, 2012).
Disease or proximate mechanisms:
Regardless of the mechanism used to establish this parasitic relationship between H. sapiens and A. lumbricoides, the question to be elucidated here is whether A. suum and A. lumbricoides are distinct species. What is clear is that the association over time has generated evolutionary drivers of parasite colonization and the host’s antagonistic response against this colonization. In Ascaris spp. infection (Girgis et al., 2013), the host uses both innate and adaptive responses as a defense against this invasion. The former response is expressed by granulocyte cells, macrophages and active metabolites (innate), while the latter uses an adaptive response with a set of regulatory cells, expressed by Th2-Treg cells, Regulatory B (Breg)(McSorley and Maizels, 2012; Turner et al., 2005). The establishment of intestinal resistance in the host is directly associated with this Th2 response, which has been demonstrated in murine models (Turner et al., 2005). Once larvae enter the bloodstream, an immune response is initiated by dendritic cells (DC) through Toll-like receptor (TLR) recognition of parasite antigens (cuticulin proteins, ascarosides and a plethora of protein structures, such as ABA-1-NPAs) and by presentation of MCH II antigens, which induce differentiation of CD4 Th2 cells. These promote the humoral secretion of several cytokine-interleukins (IL-10, IL-4,5,9 and IL-13) (Figure 2). This complex mechanism causes Th2 to activate, amplify, and maintain key innate effectors such as mast cells, basophils, eosinophils, and macrophages alternatively activated to eliminate the parasite. The expression of phenotypes through polymorphic genes of the effector subset of Th1 (IFN-γ) and Th17 cells via activation of DC presentation is responsible for the most relevant mechanisms associated with inflammation, pathology and parasite expulsion, the latter especially at the level of the intestinal lumen (Turner et al., 2005).
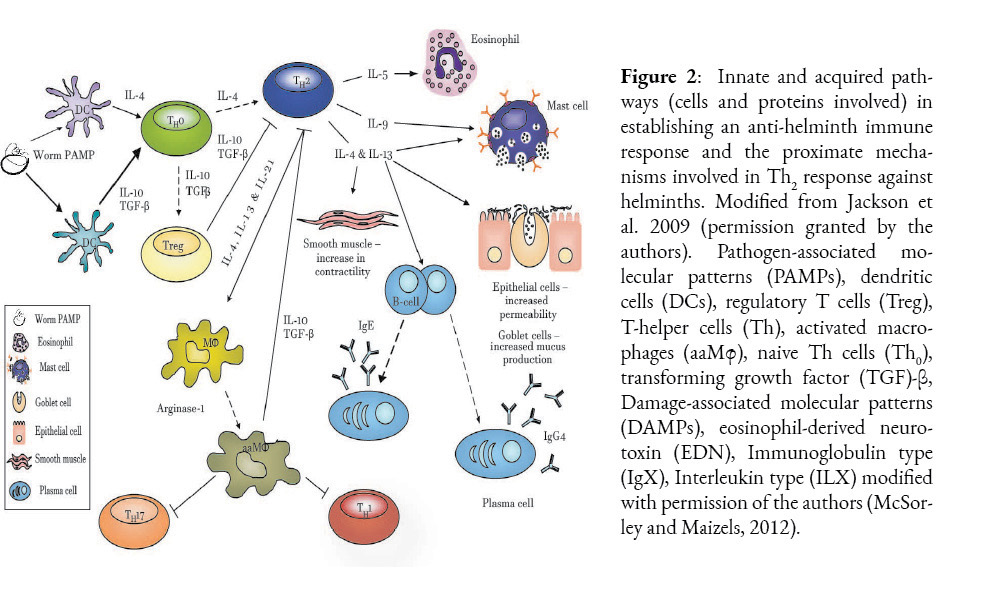
These responses are highly antagonized by Th2 cells (a source of IL-4) and Treg cells (a source of IL-10 and transforming growth factor beta (TGF-β) in the case of helminthiasis) (Holland and Smith, 2005). The parasite load in the host is not necessarily related to a higher pathology. Owing to the response of the immune system is paradoxical, as there are asymptomatic individuals with a strong regulatory response while chronic disease is observed in affected animals with low levels of infection. The asymptomatic type response is mainly regulated by IL-10 and TGF-β (CD4+Treg1), down-regulating Th1 and Th2 responses, whereas the acute type response is largely regulated by Th2 suite cells and granulocytes (Kayes, 2005; Maizels and McSorley, 2016).
On the other hand, the humoral response is associated with activated B cells (Breg) in the presence of A. lumbricoides antigens or alternatively by Th2 activation. This B effector expresses immunoglobulins of various types, notably IgE and IgG (Holland and Smith, 2005). These appear to offer some degree of protection by initiating an allergic-type reaction against the Ascaris, as well as Th2 amplifiers with effector mechanisms to clear the infection. In mouse models infected with Heligmosomoides polyrus, there is a suppression of the immune system that prevents encephalomyelitis and allergies in mice transplanted with Breg cells from parasitized mice (Jackson et al., 2009). This effect is attributed to IL-10 production and indirectly to Treg cell activation. These cell types are the main down-regulators of the immune system in the presence of helminths (Dold and Holland, 2011; Jackson et al., 2009; Maizels and McSorley, 2016). Treg cells are associated with a tolerance effect, triggering a series of linked events that promote, reinforce, and maintain Th2 pathways during infection (Allen and Maizels, 2011). The main cell phenotypes responsible for that tolerance effect are “natural” Treg, which express the cytokine FoxP3 after thymus selection, “induced” Treg, which switch to Foxp3 at the peripheral level, and Foxp3-type 1 regulatory cells. All these cells are the major expression of IL-10 and TGF-β at different levels (Girgis et al., 2013; Holland and Smith, 2005; McSorley and Maizels, 2012). These two cytokines are the principal regulators of the host response against helminths, as they enable the switch from IgE (pro-allergic/inflammatory) to IgG (non-inflammatory) isotype in chronic infections. Evidence suggests that helminths are partially responsible for this mechanism, as the administration of an anthelmintic treatment to affected patients causes a dramatic drop in circulating IgG level (Jackson et al., 2009). This would explain, to some extent, this apparent contradictory response of the host immune system to the parasite (Maizels and McSorley, 2016).
Finally, susceptibility to A. lumbricoides has been described in human family groups and may be associated with genetic factors. Studies in Nigerian children’s populations, for example, suggest an association between susceptibility to the parasite and the presence of major histocompatibility complex (MHC) resistance alleles. However, it is difficult to select which genes to study since there is not a clear identification of which are the ideal ones, leading to contradictory results such as variation on genetics and environmental factors associated with the worm burden (Williams-Blangero et al., 1999). A longitudinal study that followed subjects over a two-year period showed that in a population of 1261 people from Nepal with a known pedigree, a strong relationship between genetic factors (genes: IL7 and activated B-cell factor 1), as well as susceptibility to A. lumbricoides, was established (see details in Williams-Blangero et al 1999). This robust study demonstrated a correlation between the phenotype of human groups and helminth egg load and concluded that between 30% (p=0.0016) and 50% (p<0.0001) of the variation in worm burden was due to genetic factors. This was the first study to establish the importance of host genetic factors in determining parasite load, demonstrating the urgency of determining which genes are responsible for causing this susceptibility (Williams-Blangero et al., 2013).
Parasite evasion mechanisms:
Vertebrates have evolved a complex immune response to defend themselves against various micro and macroparasites, which focuses on keeping the parasite under control. This demonstrates the complex process of co-evolution between hosts and parasites (Schmid-Hempel, 2009). Throughout the evolutionary history between host and parasite, the immune system has carried out an innate immune response according to the type of threat (pathogens). However, roundworms have evolved several mechanisms to evade or regulate the host immune system to ensure their coexistence. Ascaris infections are usually chronic, with a low pathogenic effect on the host (lower growth rate and lower capacity for damage in target organs), which implies a level of adaptation in the process of co-evolution with their host (Jackson et al. 2009). A high prevalence of these parasites can be seen in humans and pigs in endemic zones (Dold and Holland, 2011). Some of the strategies of ascarids to remain in their host are through the expression of genes encoding molecules such as TGF-β-like ligand, glycoproteins, phosphorylcholines together with ES-62 N-type glycans by down-modulation, subversion or evasion of the host immune system (Girgis et al., 2013). These mechanisms, although not yet well understood, allow the parasite to complete its life cycle by reaching sexual maturity, reproducing in its host and leaving offspring for the species to survive.
Immune tolerance trade-off:
The concept of co-evolution is reflected in the host-parasite’s own mechanisms and their constant adjustment and imbalance as an evolutionary response to a strategy aimed at reinforcing the immune defense system (receptors, molecular mediators, signaling pathways) over time (Dold and Holland, 2011; Jackson et al., 2009; Maizels and McSorley, 2016). Thus, vertebrates developed an immune system that anticipated encounters (due to the ubiquitous nature of helminths) with parasites that stimulated the Th2/Treg response (Jackson et al., 2009; Maizels, 2009).
The complex response of the immune system to helminths echoes the diversification of methods and redundancy in some of them to develop and strengthen mechanisms of resistance to the presence of ascarids. For example, the Th2-mediated response is mimicked or reinforced by a wide array of helper cells in the innate response by secreting chemical mediators that further stimulate the Th2 response in helminth infections, or by overlapping the cytokine-producing function of other cells (especially non-B or T cells to perform CD4+Th2-like functions) (Allen and Maizels, 2011; Maizels and McSorley, 2016). There is no evidence that nematode expulsion or death occurs exclusively by granulocyte action; rather it is possibly associated with the energetic cost, as the activation of this innate response and the associated high cost of controlling this tissue pathology overcomes the benefit (Maizels, 2009; Turner et al., 2008). It seems that in this co-evolutionary process between host and parasite, the host established a mechanism to apply a more reasonable strategy. This is based on resistance-tolerance to the parasite, ensuring the maintenance of immune homeostasis at an acceptable cost-benefit to achieve optimal fitness. These two antagonistic processes (resistance-tolerance) occur simultaneously in the host due to this homeostatic balance. Resistance is expressed in the mechanisms through Th2 cells (immune regulation, repair, and anti-parasitic effect), while tolerance is expressed in complex mechanisms targeting the active suppression of autoreactive Treg cells mediated by Treg cells as an essential mechanism of self-tolerance and B-cell action (Allen and Maizels, 2011; Johnston et al., 2014). H. sapiens populations possess extensive polymorphism in immune-associated genes that have evolved to positively select those alleles that express an “optimal” response to the parasite (tolerance-resistance). Interestingly, parasite tolerance may be favored in children born to parasite-infected mothers (uterine tolerance), being more susceptible to infections at a younger age by showing a lower T-cell response to parasite antigen than those born to parasite-free mothers(Maizels and McSorley, 2016). Unfortunately, the ineffective memory response of the host immune system to the parasite is a product of this damping effect. This has been demonstrated in mouse models of helminth infection and in human ascariasis, where after total parasite elimination by anthelmintic therapy, subjects are susceptible to parasites in the absence of a memory immune response (Maizels, 2009). In addition, the immune system is unable to maintain a Th2/Treg response and a downmodulated cytokine production (IL-10) in the absence of the parasite stimulus, which may explain some of the increased incidence of allergies and autoimmune diseases in developed countries (Jackson et al., 2009; McSorley and Maizels, 2012). High single nucleotide polymorphism (SNP) frequencies were determined in an analysis of ~100 human interleukin genes. In geographic areas with a high helminth presence, these loci show high variability in encoding cytokines for both innate and adaptive responses (Maizels, 2009). The low incidence of immunopathology in endemic countries with high prevalence of helminths (including ascarids) is associated with a genetic locus that increases the probability of developing tolerance against the parasite in developing countries. This selection is evident, for example, in a non-coding variant allele of the STAT-6 regulatory transcript that is part of the IL-4 pathway, which is associated with increased incidence of asthma due to the absence of infection by ascariasis and reduced susceptibility against A. lumbricoides in humans (Maizels, 2009). This evidence suggests that there was an evolutionary mismatch in the compensation trade-off.
Ascarid strategy:
The immune system has evolved by constant helminth infestation; as a result, helminths (including ascarids) have counteracted the host, evolving damping processes rather than evading or deactivating the host immune system response (McSorley and Maizels, 2012). In this trade-off, the parasite reinforces the host’s strategy to tolerate ascarids by producing molecules that mimic endogenous host mechanisms, which, in turn, control immunosuppression. For example, blockade of TLR receptors, which are used by other molecules such as lipopolysaccharides as an inflammatory response, is a phenomenon observed in mice (Apodemus sylvaticus), which are infected by H. polygyrus under natural conditions (Maizels, 2009). This may partly explain the parasite’s strategy to stimulate host tolerance. Although nematode defense mechanisms are still poorly understood, it is known that the helminth cuticle can stimulate a complement-mediated inflammatory response via the alternative pathway. The complement antagonistic response of the parasite occurs through the secretion of molecules on the surface cuticle ES-62 (Coakley et al., 2016). In turn, Breg cells are part of this down-regulation, by being directly stimulated by helminths and expressing IL-10, a phenomenon observed in rodent models (McSorley and Maizels, 2012).
Anthelmintic resistance:
In human ascariasis, strategies to control endemic populations are to reduce parasite intensity through improved health conditions, environmental sanitation, health information, and anthelmintic chemotherapy (Dold and Holland, 2011; Holland and Smith, 2005). Benzimidazole derivatives (albendazole) bind to β-tubulin and block tubulin polymerization, thereby destabilizing helminth microtubules. These drugs have been used in mass drug campaigns in endemic areas such Nigeria or Nepals (Leung et al., 2020); however, this could lead to their resistance (Diawara et al., 2009; Holland and Smith, 2005; Krücken et al., 2017).
Evidence of rodent models with human parasites have identified the emergence of helminths with resistance to these drugs, which is associated with resistance alleles (F167Y, E198A, F200Y) (Churcher and Basanez, 2008; Dilks et al., 2020). In Trichocephalus trichiurus, resistance is associated with a mutation in the SNP responsible for a substitution of the amino acid Phenylalanine for Tyrosine at codon parasite 200 TAC mutant type SNP. It is unclear whether this resistance is recessive, semi-dominant, or dominant (Diawara et al., 2009). The question, therefore, arises as to whether there will be a positive selection force of these genes in populations under constant pressure from this drug as a parasite evolutionary mechanism (Churcher and Basanez, 2008). Mathematical modeling showing the global decrease in parasite load on the host evidences the negative impact of density-dependent processes on natural selection on resistance genes. Findings suggest that the use of anthelmintic therapies leads to parasite depletion in the host, releasing the pressure of negative density-dependent mechanisms on the surviving parasites (Churcher and Basanez, 2008). This increases the likelihood of transmission of resistance alleles in the progeny. In addition, the possibility of host-to-host transmission and the generation of hybrids of A. suum and A. lumbricoides may lead to a process of introgression with the expression of a deworming resistant phenotype. This may favor an increased virulence of the parasite or gene flow and thus the transfer of resistance alleles (Betson et al., 2014; Criscione et al., 2007).
Horizon:
Multiple rodent models of helminth parasitosis have proven the association between the parasite and its down-regulatory mechanism of the immune system, leading to an antigen-specific anergic stage, which results in a suppression of symptoms in pathologically induced mice (Zhou et al., 2011). Protective effects of A. lumbricoides and other gastrointestinal helminths against multiple sclerosis have been described in accidentally infected sclerotic patients (Correale, 2014). This damping effect does not always have positive consequences for the host. Host compensation can generate negative effects (Figure 2) over the interaction of the nematode (for example Schistosoma haematobium) with the host immune response. That effect is a bioproduct that contributes to a positive carcinogenic outcome favoring the growth of cancer cells through the production of cancer promoters by the parasite as a result of this damping or by reducing the efficacy of vaccines or increasing susceptibility to other infections or other microparasites such as the influenza virus (Casado-Maestre et al., 2011; Johnston et al., 2014; McSorley and Maizels, 2012; Schneider-Crease et al., 2021). In this context, the proximity mechanism, as understood from the evolutionary point of view, can give a new horizon for a better mechanism in disease therapies.
Therapy drawback:
Much of the controversy surrounding live parasite therapies as a treatment for autoimmune conditions and associated diseases in humans is based on findings associated with the paradoxical effects of this relationship (old friend and hygiene hypothesis) (Frew, 2019). On one hand, their true efficacy is questioned, as they are based on animal-parasite models that do not reflect the genuine response expected in humans. On the other hand, studies show little or no efficacy of parasites in reducing these type of diseases (Briggs et al., 2016; Wammes et al., 2014). Multiple studies emphasizes, rather, the high risk that exists with the parasitic loads used and the pernicious effects of this iatrogenic parasitosis (Briggs et al., 2016). Even more critical is the fact that several studies suggest a rather opposite effect to the proposed protective result, lighting the increased allergic susceptibility of the host due to helminthic infections (Wammes et al., 2014). However, this conflicting evidence is based on the results of studies with a certain degree of bias due to the animals or mathematical models used. This stresses the need for a more accurate model. There are multiple factors such as burden, host genetic, parasitosis chronicity, or mix factors that may mask the true beneficial effect of helminths and their evolving host-parasite relationship. Future research should include aspects such as the role of parasite ecology and the difference in cultural and epigenetic patterns to try to give a more accurate explanation of the benefit or this type of future therapy (Kondrashova et al., 2013; Wammes et al., 2014; Yang y Schwartz, 2012). Prioritizing studies that focus on parasite bioproducts (proteins, lipids, etc.) that could modulate the immune-metabolic pathway for future and safer therapies should be the aim of new experiments and treatments (van Kruiningen and West, 2005).
Final remarks
Parasitic diseases have been associated with human beginnings, and this relationship between both host and parasite has developed over time. Evolutionary medicine believes in a strong link between evolutive and proximate mechanisms, giving an accurate explanation for the tradeoff associated with diseases and the relationship between pathogens (macro and micro parasite) and their hosts. Ascariasis in humans caused by Ascaris lumbricoides or A. suum is a good example in which both mechanisms can be explored and illustrates the effect of how such mechanism should be better understood under the evolution perspective.
Using an evolutionary approach, immunological mechanisms responsible for the damping process and host-parasite interactions could be understood in detail and give guidance for better therapies to avoid negative evolutionary mismatch. The identification of precise genetic variants could develop future therapies, including the manipulation of disease mechanisms to control autoimmune diseases and allergies, the prevention of allographic rejection, and a better targeting of control methods of this parasitosis in the near future (Johnston et al., 2014).
A better understanding of how evolution is part of modern medicine is paramount to having a more advanced human and animal medicine, without losing sight of the powerful influence that evolution has on health.
References
Allen, J. E. y Maizels, R. M. (2011). Diversity and dialogue in immunity to helminths. Nature Reviews. Immunology, 11(6), 375–388. https://doi.org/10.1038/nri2992
Anderson, T. J. C [T. J. C.], Romero-Abal, M. E. y Jaenike, J. (1993). Genetic structure and epidemiology of Ascaris populations: Patterns of host affiliation in Guatemala. Parasitology, 107(03), 319. https://doi.org/10.1017/S0031182000079294
Anderson, T. J. (2001). The dangers of using single locus markers in parasite epidemiology: Ascaris as a case study. Trends in Parasitology, 17(4), 183–188. https://doi.org/10.1016/s1471-4922(00)01944-9
Barendregt, J. J. (2002). Disability-adjusted Life Years (DALYs) and Disability-adjusted Life Expectancy (DALE). En J.-M. Robine, C. Jagger, C. D. Mathers, E. M. Crimmins y R. M. Suzman (Eds.), Determining Health Expectancies (pp. 247–261). John Wiley & Sons, Ltd. https://doi.org/10.1002/0470858885.ch13
Betson, M [Martha], Nejsum, P [Peter], Bendall, R. P [Richard P.], Deb, R. M. y Stothard, J. R [J. Russell] (2014). Molecular epidemiology of ascariasis: A global perspective on the transmission dynamics of Ascaris in people and pigs. The Journal of Infectious Diseases, 210(6), 932–941. https://doi.org/10.1093/infdis/jiu193
Briggs, N., Weatherhead, J., Sastry, K. J. y Hotez, P. J. (2016). The Hygiene Hypothesis and Its Inconvenient Truths about Helminth Infections. PLoS Neglected Tropical Diseases, 10(9), e0004944. https://doi.org/10.1371/journal.pntd.0004944
Casado-Maestre, M. D., Alamo-Martínez, J. M., Segura-Sampedro, J. J., Durán-Izquierdo, E., Marín-Gómez, L. M., Bernal-Bellido, C., Suárez-Artacho, G., Serrano-Díez-Canedo, J., Gómez-Bravo, M. Á. y Padillo-Ruiz, F. J. (2011). Ascaris lumbricoides as etiologic factor for pancreas inflammatory tumor. Revista Espanola De Enfermedades Digestivas : Organo Oficial De La Sociedad Espanola De Patologia Digestiva, 103(11), 592–593. https://doi.org/10.4321/S1130-01082011001100008
Cavallero, S., Snabel, V., Pacella, F., Perrone, V., D’Amelio, S. y Zhou, X.-N. (2013). Phylogeographical Studies of Ascaris spp. Based on Ribosomal and Mitochondrial DNA Sequences. PLoS Neglected Tropical Diseases, 7(4), e2170. https://doi.org/10.1371/journal.pntd.0002170
Churcher, T. S. y Basanez, M.-G. (2008). Density dependence and the spread of anthelmintic resistance. Evolution; International Journal of Organic Evolution, 62(3), 528–537. https://doi.org/10.1111/j.1558-5646.2007.00290.x
Coakley, G., Buck, A. H. y Maizels, R. M. (2016). Host parasite communications-Messages from helminths for the immune system: Parasite communication and cell-cell interactions. Molecular and Biochemical Parasitology, 208(1), 33–40. https://doi.org/10.1016/j.molbiopara.2016.06.003
Correale, J. (2014). Helminth/parasite treatment of multiple sclerosis. Current Treatment Options in Neurology, 16(6), 296. https://doi.org/10.1007/s11940-014-0296-3
Criscione, C. D., Anderson, J. D., Sudimack, D., Peng, W., Jha, B [Bharat], Williams-Blangero, S [Sarah] y Anderson, T. J. C [Timothy J. C.] (2007). Disentangling hybridization and host colonization in parasitic roundworms of humans and pigs. Proceedings. Biological Sciences, 274(1626), 2669–2677. https://doi.org/10.1098/rspb.2007.0877
Dall’ Orso, P, Cantou,V., Rosano K.,, De los Santos, K., Fernández,N., Berazategui,R., Giachetto, R. (2014). Ascaris lumbricoides. Complicaciones graves en niños hospitalizados en el Centro Hospitalario Pereira Rossell. Archivos De Pediatría Del Uruguay, 3(85), 149–154. http://www.scielo.edu.uy/pdf/adp/v85n3/v85n3a02.pdf
Diawara, A., Drake, L. J., Suswillo, R. R., Kihara, J., Bundy, D. A. P., Scott, M. E., Halpenny, C., Stothard, J. R [J. Russell] y Prichard, R. K. (2009). Assays to detect beta-tubulin codon 200 polymorphism in Trichuris trichiura and Ascaris lumbricoides. PLoS Neglected Tropical Diseases, 3(3), e397. https://doi.org/10.1371/journal.pntd.0000397
Dilks, C. M., Hahnel, S.R., Sheng, Q., Long, L., McGrath, P. T. y Andersen, E. C. (2020). Quantitative benzimidazole resistance and fitness effects of parasitic nematode beta-tubulin alleles. International Journal for Parasitology. Drugs and Drug Resistance, 14, 28–36. https://doi.org/10.1016/j.ijpddr.2020.08.003
Dold, C. y Holland, C.V [Celia V.] (2011). Ascaris and ascariasis. Microbes and Infection, 13(7), 632–637. https://doi.org/10.1016/j.micinf.2010.09.012
Dunsworth, H. M. (2010). Origin of the Genus Homo. Evolution: Education and Outreach, 3(3), 353–366. https://doi.org/10.1007/s12052-010-0247-8
Easton, A., Gao, S., Lawton, S.P., Bennuru, S., Khan, A., Dahlstrom, E., Oliveira, R.G., Kepha, S., Porcella, S. F., Webster, J., Anderson, R., Grigg, M. E., Davis, R. E., Wang, J. y Nutman, T. B. (2020). Molecular evidence of hybridization between pig and human Ascaris indicates an interbred species complex infecting humans. ELife, 9. https://doi.org/10.7554/eLife.61562
Frew, J. W. (2019). The Hygiene Hypothesis, Old Friends, and New Genes. Frontiers in Immunology, 10, 388. https://doi.org/10.3389/fimmu.2019.00388
Girgis, N. M., Gundra, U. M., Loke, P. y Knoll, L. J. (2013). Immune Regulation during Helminth Infections. PLoS Pathogens, 9(4), e1003250. https://doi.org/10.1371/journal.ppat.1003250
Holland, C. V [C. V.] y Smith, H. V. (Eds.). (2005). Toxocara: the enigmatic parasite. CABI. https://doi.org/10.1079/9781845930264.0000
Holt, R. D., Dobson, A. P., Begon, M., Bowers, R. G. y Schauber, E. M. (2003). Parasite establishment in host communities. Ecology Letters, 6(9), 837–842. https://doi.org/10.1046/j.1461-0248.2003.00501.x
Jackson, J. A., Friberg, I. M., Little, S. y Bradley, J. E. (2009). Review series on helminths, immune modulation and the hygiene hypothesis: Immunity against helminths and immunological phenomena in modern human populations: Coevolutionary legacies? Immunology, 126(1), 18–27. https://doi.org/10.1111/j.1365-2567.2008.03010.x
Johnston, C. J. C., McSorley, H. J., Anderton, S. M., Wigmore, S. J. y Maizels, R. M. (2014). Helminths and immunological tolerance. Transplantation, 97(2), 127–132. https://doi.org/10.1097/TP.0b013e3182a53f59
Kayes, S. G. (2005). Inflammatory and immunological responses to Toxocara canis. En C. V. Holland y H. V. Smith (Eds.), Toxocara: the enigmatic parasite (pp. 158–173). CABI. https://doi.org/10.1079/9781845930264.0158
Kondrashova, A., Seiskari, T., Ilonen, J., Knip, M. y Hyöty, H. (2013). The ‘Hygiene hypothesis’ and the sharp gradient in the incidence of autoimmune and allergic diseases between Russian Karelia and Finland. APMIS, 121(6), 478–493. https://doi.org/10.1111/apm.12023
Krücken, J., Fraundorfer, K., Mugisha, J. C., Ramünke, S., Sifft, K. C., Geus, D., Habarugira, F., Ndoli, J., Sendegeya, A., Mukampunga, C., Bayingana, C., Aebischer, T., Demeler, J., Gahutu, J. B., Mockenhaupt, F. P. y Samson-Himmelstjerna, G. von (2017). Reduced efficacy of albendazole against Ascaris lumbricoides in Rwandan schoolchildren. International Journal for Parasitology. Drugs and Drug Resistance, 7(3), 262–271. https://doi.org/10.1016/j.ijpddr.2017.06.001
Larson, G., Dobney, K., Albarella, U., Fang, M., Matisoo-Smith, E., Robins, J., Lowden, S., Finlayson, H., Brand, T., Willerslev, E., Rowley-Conwy, P., Andersson, L. y Cooper, A. (2005). Worldwide phylogeography of wild boar reveals multiple centers of pig domestication. Science (New York, N.Y.), 307(5715), 1618–1621. https://doi.org/10.1126/science.1106927
Leles, D., Gardner, S. L., Reinhard, K., Iniguez, A. y Araujo, A. (2012). Are Ascaris lumbricoides and Ascaris suum a single species? Parasites & Vectors, 5, 42. https://doi.org/10.1186/1756-3305-5-42
Leung, A.K.C., Leung, A.A.M., Wong, A.H.C. y Hon, K. L. (2020). Human Ascariasis: An Updated Review. Recent Patents on Inflammation & Allergy Drug Discovery, 14(2), 133–145. https://doi.org/10.2174/1872213X14666200705235757
Liu, G.-H., Wu, C.-Y., Song, H.-Q., Wei, S.-J., Xu, M.-J., Lin, R.-Q., Zhao, G.-H., Huang, S.-Y. y Zhu, X.-Q. (2012). Comparative analyses of the complete mitochondrial genomes of Ascaris lumbricoides and Ascaris suum from humans and pigs. Gene, 492(1), 110–116. https://doi.org/10.1016/j.gene.2011.10.043
Loreille, O. y Bouchet, F. (2003). Evolution of ascariasis in humans and pigs: A multi-disciplinary approach. Memórias Do Instituto Oswaldo Cruz, 98(suppl 1), 39–46. https://doi.org/10.1590/S0074-02762003000900008
Maizels, R.M. (2009). Parasite immunomodulation and polymorphisms of the immune system. Journal of Biology, 8(7), 62. https://doi.org/10.1186/jbiol166
Maizels, R.M. y McSorley, H.J. (2016). Regulation of the host immune system by helminth parasites. The Journal of Allergy and Clinical Immunology, 138(3), 666–675. https://doi.org/10.1016/j.jaci.2016.07.007
McSorley, H.J. y Maizels, R.M. (2012). Helminth infections and host immune regulation. Clinical Microbiology Reviews, 25(4), 585–608. https://doi.org/10.1128/CMR.05040-11
Nadler, S. A. y Hudspeth, D. S. (2000). Phylogeny of the Ascaridoidea (Nematoda: Ascaridida) based on three genes and morphology: Hypotheses of structural and sequence evolution. The Journal of Parasitology, 86(2), 380–393. https://doi.org/10.1645/0022-3395(2000)086[0380:POTANA]2.0.CO;2
Nejsum, P [P.], Betson, M [M.], Bendall, R. P [R. P.], Thamsborg, S.M. y Stothard, J.R [J. R.] (2012). Assessing the zoonotic potential of Ascaris suum and Trichuris suis: Looking to the future from an analysis of the past. Journal of Helminthology, 86(2), 148–155. https://doi.org/10.1017/S0022149X12000193
Nejsum, P [Peter], Hawash, M. B. F., Betson, M [Martha], Stothard, J. R [J. Russell], Gasser, R. B. y Andersen, L.O. (2017). Ascaris phylogeny based on multiple whole mtDNA genomes. Infection, Genetics and Evolution : Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases, 48, 4–9. https://doi.org/10.1016/j.meegid.2016.12.003
O’Lorcain, P. y Holland, C. V [C. V.] (2000). The public health importance of Ascaris lumbricoides. Parasitology, 121 Suppl, S51-71. https://doi.org/10.1017/S0031182000006442
Parker, W. y Ollerton, J. (2013). Evolutionary biology and anthropology suggest biome reconstitution as a necessary approach toward dealing with immune disorders. Evolution, Medicine, and Public Health, 2013(1), 89–103. https://doi.org/10.1093/emph/eot008
Peng, W. y Criscione, C. D. (2012). Ascariasis in people and pigs: New inferences from DNA analysis of worm populations. Infection, Genetics and Evolution : Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases, 12(2), 227–235. https://doi.org/10.1016/j.meegid.2012.01.012
Peng, W., Yuan, K., Zhou, X., Hu, M., Abs EL-Osta, Y. G. y Gasser, R. B. (2003). Molecular epidemiological investigation of Ascaris genotypes in China based on single-strand conformation polymorphism analysis of ribosomal DNA. Electrophoresis, 24(14), 2308–2315. https://doi.org/10.1002/elps.200305455
Rito, T., Vieira, D., Silva, M., Conde-Sousa, E., Pereira, L., Mellars, P., Richards, M. B. y Soares, P. (2019). A dispersal of Homo sapiens from southern to eastern Africa immediately preceded the out-of-Africa migration. Scientific Reports, 9(1), 4728. https://doi.org/10.1038/s41598-019-41176-3
Roberts, L. S., Schmidt, G. D. y Janovy, J. (op. 2009). Gerald D. Schmidt & Larry S. Roberts’ foundations of parasitology (8th edition). McGraw-Hill Higher Education.
Schmid-Hempel, P. (2009). Immune defence, parasite evasion strategies and their relevance for ‘macroscopic phenomena’ such as virulence. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364(1513), 85–98. https://doi.org/10.1098/rstb.2008.0157
Schneider-Crease, I. A., Blackwell, A. D., Kraft, T. S., Emery Thompson, M., Maldonado Suarez, I., Cummings, D. K., Stieglitz, J., Snyder-Mackler, N., Gurven, M., Kaplan, H. y Trumble, B. C. (2021). Helminth infection is associated with dampened cytokine responses to viral and bacterial stimulations in Tsimane forager-horticulturalists. Evolution, Medicine, and Public Health, 9(1), 349–359. https://doi.org/10.1093/emph/eoab035
Turner, J. D., Faulkner, H., Kamgno, J., Kennedy, M. W., Behnke, J., Boussinesq, M. y Bradley, J. E. (2005). Allergen-specific IgE and IgG4 are markers of resistance and susceptibility in a human intestinal nematode infection. Microbes and Infection, 7(7-8), 990–996. https://doi.org/10.1016/j.micinf.2005.03.036
Turner, J. D., Jackson, J. A., Faulkner, H., Behnke, J., Else, K. J., Kamgno, J., Boussinesq, M. y Bradley, J. E. (2008). Intensity of intestinal infection with multiple worm species is related to regulatory cytokine output and immune hyporesponsiveness. The Journal of Infectious Diseases, 197(8), 1204–1212. https://doi.org/10.1086/586717
van Kruiningen, H. J. y West, A. B. (2005). Potential danger in the medical use of Trichuris suis for the treatment of inflammatory bowel disease. Inflammatory Bowel Diseases, 11(5), 515.
Wammes, L.J., Mpairwe, H., Elliott, A.M. y Yazdanbakhsh, M. (2014). Helminth therapy or elimination: Epidemiological, immunological, and clinical considerations. The Lancet Infectious Diseases, 14(11), 1150–1162. https://doi.org/10.1016/S1473-3099(14)70771-6
Williams-Blangero, S [S.], Subedi, J., Upadhayay, R.P., Manral, D.B., Rai, D.R., Jha, B [B.], Robinson, E. S. y Blangero, J [J.] (1999). Genetic analysis of susceptibility to infection with Ascaris lumbricoides. The American Journal of Tropical Medicine and Hygiene, 60(6), 921–926.
Williams-Blangero, S [Sarah], Fenstad, M.H., Kumar, S. y Blangero, J [John]. (2013). Genetics of Human Host Susceptibility to Ascariasis. En Ascaris: The Neglected Parasite (pp. 315–340). Elsevier. https://doi.org/10.1016/B978-0-12-396978-1.00012-4
Yang, I.V. y Schwartz, D.A. (2012). Epigenetic mechanisms and the development of asthma. The Journal of Allergy and Clinical Immunology, 130(6), 1243–1255. https://doi.org/10.1016/j.jaci.2012.07.052
Zhou, C., Li, M., Yuan, K., Hu, N. y Peng, W. (2011). Phylogeography of Ascaris lumbricoides and A. Suum from China. Parasitology Research, 109(2), 329–338. https://doi.org/10.1007/s00436-011-2260-4

Licencia Creative Commons Atribución-No-Comercial